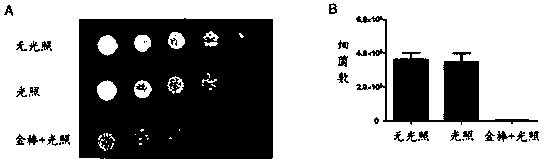
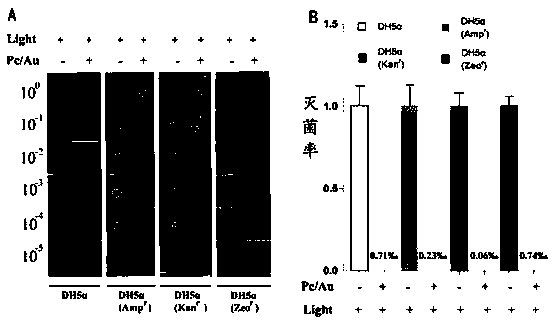
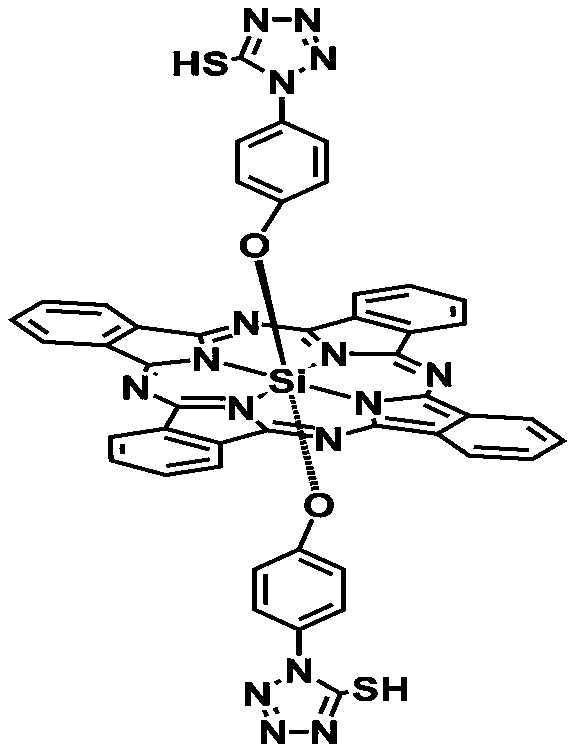
Matryoshka doll-type gold nanorod composite system loaded with novel sulfydryl N-heterocyclic silicon (IV) phthalocyanine complex
A technology of silicon phthalocyanine and nano-gold rods, which is applied in the field of nano-biomedicine, can solve the problems of reduced fluorescence lifetime, singlet oxygen quantum yield, reduced photodynamic therapy effect, and high phototoxicity, so as to increase singlet oxygen quantum yield efficiency, fluorescence intensity enhancement, and the effect of isolation protection enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] Below in conjunction with embodiment the present invention is described in detail:
[0028] 1) Preparation of dichlorosilicon (IV) phthalocyanine
[0029] Add 7.28g of 1,3-diiminoisoindoline and 83mL of quinoline into the three-neck flask. After gradually raising the temperature to 60°C, add 8.3mL of silicon tetrachloride, raise the temperature to 220°C, react for 30min, and cool to At room temperature, the reactant was poured into 200 mL of methanol solution, stirred ultrasonically for 30 min, allowed to stand for 1 h, then filtered with suction, washed with solvents such as acetone, methanol, dichloromethane, acetone, etc. until the supernatant was clear. After drying, 4.1256 g of blue-purple powdery solid (1) was obtained, with a yield of 49.2%.
[0030] 2) Preparation of di-(1-(4-hydroxyphenyl)-5-mercapto-tetrazolyl) axially substituted silicon (IV) phthalocyanine
[0031] In a round bottom flask was added 1-(4-hydroxyphenyl)-5-mercapto-tetrazolium (0.0611 g), 1-(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com