Organic light-emitting molecule and application thereof in organic light-emitting diode
A technology of light-emitting diodes and organic light-emitting layers, which is applied in the fields of light-emitting materials, organic chemistry, and organic chemical methods, and can solve the problems of high device driving voltage and failure to attract widespread attention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
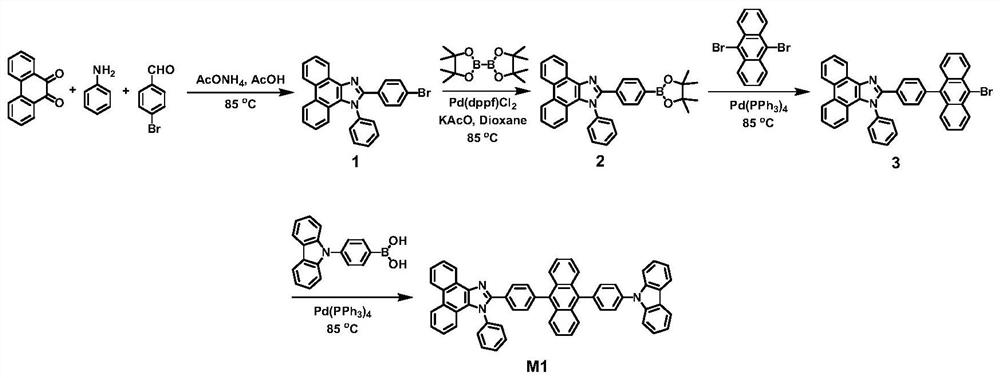
[0030] Preparation of compound M1
[0031] The structural formula and synthetic route of compound M1 are shown below, and the specific synthetic method is as follows
[0032]
[0033] (1) Synthesis of compound 1
[0034] Under nitrogen protection, phenanthrenequinone (10mmol), p-bromobenzaldehyde (10mmol) and aniline (10mmol) were added to 100ml of acetic acid, heated to reflux for 24 hours. After cooling to room temperature and standing still, suction filtration was performed, and the filter residue was washed with ethanol three times to obtain a crude product. Recrystallization with tetrahydrofuran / ethanol mixed solvent gave a white solid product with a yield of 85%. 1 H NMR, 13 The results of CNMR, MS and elemental analysis showed that the obtained compound was the target product.
[0035] (2) Synthesis of compound 2
[0036] In a nitrogen atmosphere, compound 1 (10mmol), biboronic acid pinacol ester (12 mmol), potassium acetate (10mmol), [1,1'-bis(diphenylphosphino...
Embodiment 2
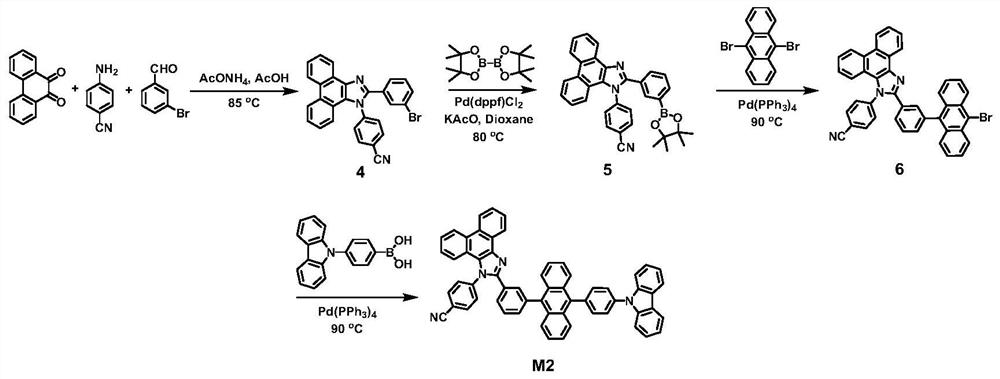
[0042] Preparation of compound M2
[0043] The structural formula and synthetic route of compound M2 are shown below, and the specific synthetic method is as follows
[0044]
[0045] (1) Synthesis of Compound 4
[0046] Under nitrogen protection, phenanthrenequinone (10mmol), m-bromobenzaldehyde (10mmol) and aniline (10mmol) were added to 100ml of acetic acid, and heated to reflux for 24 hours. After cooling to room temperature and standing still, suction filtration was performed, and the filter residue was washed with ethanol three times to obtain a crude product. Recrystallization with tetrahydrofuran / ethanol mixed solvent gave a white solid product with a yield of 86%. 1 H NMR,13 The results of CNMR, MS and elemental analysis showed that the obtained compound was the target product.
[0047] (2) Synthesis of Compound 5
[0048] In a nitrogen atmosphere, compound 4 (10mmol), biboronic acid pinacol ester (12 mmol), potassium acetate (10mmol), [1,1'-bis(diphenylphosphi...
Embodiment 3
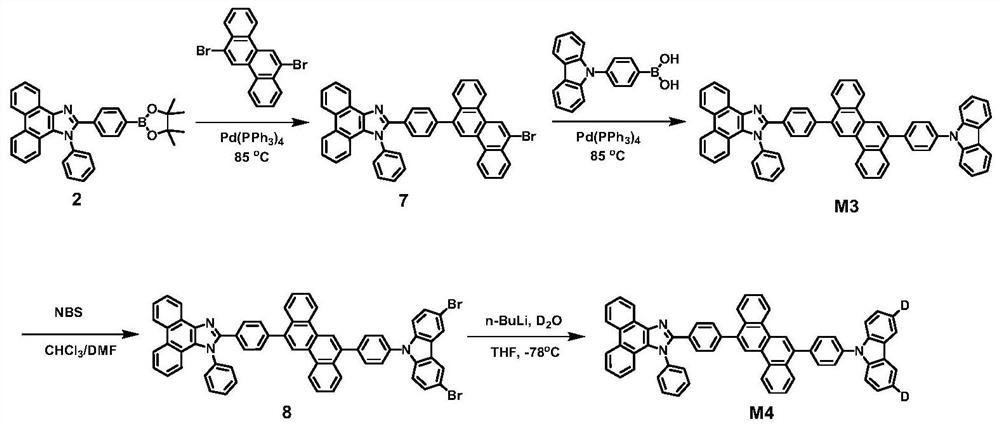
[0054] Preparation of Compounds M3 and M4
[0055] The structural formulas and synthetic routes of compounds M3 and M4 are shown below, and the specific synthetic methods are as follows
[0056]
[0057] (1) Synthesis of Compound 7
[0058] Under the protection of nitrogen, compound 2 (10mmol), 6,12-dibromide (10mmol), potassium carbonate (30mmol), tetrakis (triphenylphosphine) palladium (0.5mmol) were dissolved in 50ml of toluene and 10ml of water, and then added Tetrabutylammonium bromide was used as a phase transfer catalyst, and the temperature was raised to 85°C for 12 hours. After the reaction, the toluene solvent was distilled off under reduced pressure, the product was extracted with dichloromethane, washed three times with saturated aqueous sodium chloride solution, the organic phase was removed from the solvent using a rotary evaporator, and the crude product was purified by column chromatography, and fixed with silica gel. phase, petroleum ether / dichloromethane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com