Nanoparticle compound with tumor microenvironment responsive deformation
A combination and drug technology, applied in anti-tumor drugs, drug combinations, drug delivery, etc., can solve the problem of poor tumor site retention time, and achieve the effects of stable structure, small side effects, and high drug loading.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
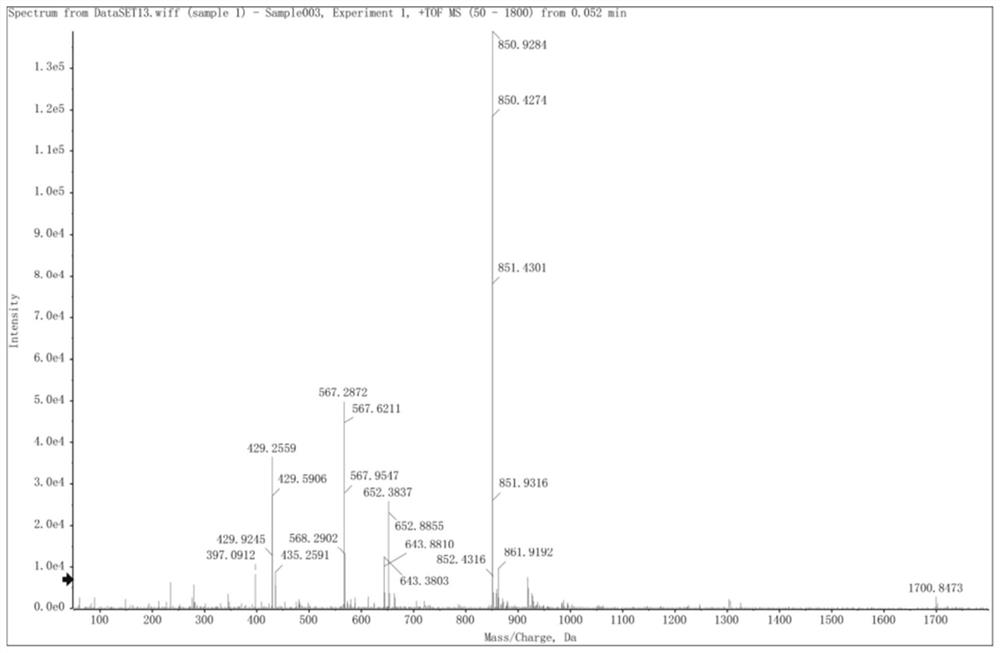
[0039] Example 1: Preparation of Amphiphilic Oligomeric Polypeptide Drug Conjugate Dox-Suc-KIGLFRWR
[0040] 1. Materials
[0041] 9-Wat methoxycarbonyl (Fmoc) protected glycine (G), Fmoc protected phenylalanine (F), Fmoc and tert-butoxycarbonyl (Boc) protected tryptophan (W), Fmoc protected leucine Amino acid (L), Fmoc and 2,2,4,6,7-pentamethyl-2H-benzofuran-5-sulfonyl (Pbf) protected arginine (R), Fmoc protected isoleucine Acid (I), Fmoc and 2-(4,4-dimethyl-2,6-dioxocyclohexylmethylene)ethyl protected lysine (K), Rink Amide-AM Resin (100 -200 mesh, substitution coefficient: 0.486mmol / g), O-benzotriazole-tetramethylurea hexafluorophosphate (HBTU, 99%); N,N-diisopropylethylamine (DIEA, 99 %); piperidine (Piperidine, 99%), N,N-dimethylformamide (DMF, 99%), triethylamine; triisopropylsilane (Tis, 99%); trifluoroacetic acid (TFA, 99%).
[0042] 2. Preparation method
[0043] Using conventional solid-phase synthesis methods, oligomeric polypeptides are synthesized, and then t...
Embodiment 2
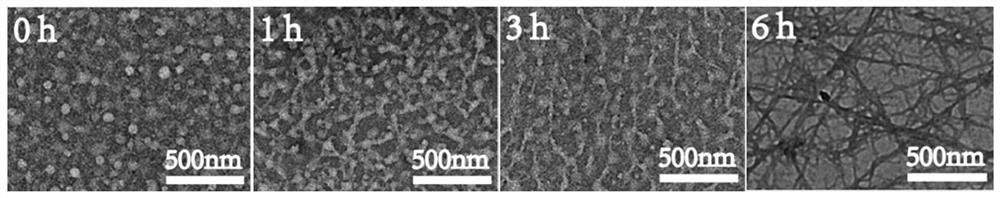
[0049] Example 2: Transmission electron microscopy (TEM) examination of Dox-Suc-KIGLFRWR self-assembly
[0050] Dox-Suc-KIGLFRWR solution with a concentration of 100 μM was prepared. After 0, 1, 6, and 12 hours of assembly, the solution was dropped onto a copper mesh coated with a support film for 60 s, and the excess solution was absorbed with filter paper. After negative staining with uranyl acetate stain for 90 s, use filter paper to absorb excess dye solution, dry naturally, and place it under a transmission electron microscope for observation, see figure 2 , from left to right are the electron microscope images of assembly 0h, 1h, 3h, and 6h, respectively. It can be seen from the figure that the peptide-drug conjugate self-assembles to form spherical micelles with a size of 40-50 nm at 0 h. After 1 h, the micelles further aggregate to form primary fibers. After 6 h, the fibers are intertwined and aggregated, indicating that the synthesized polypeptide-drug The conjugate...
Embodiment 3
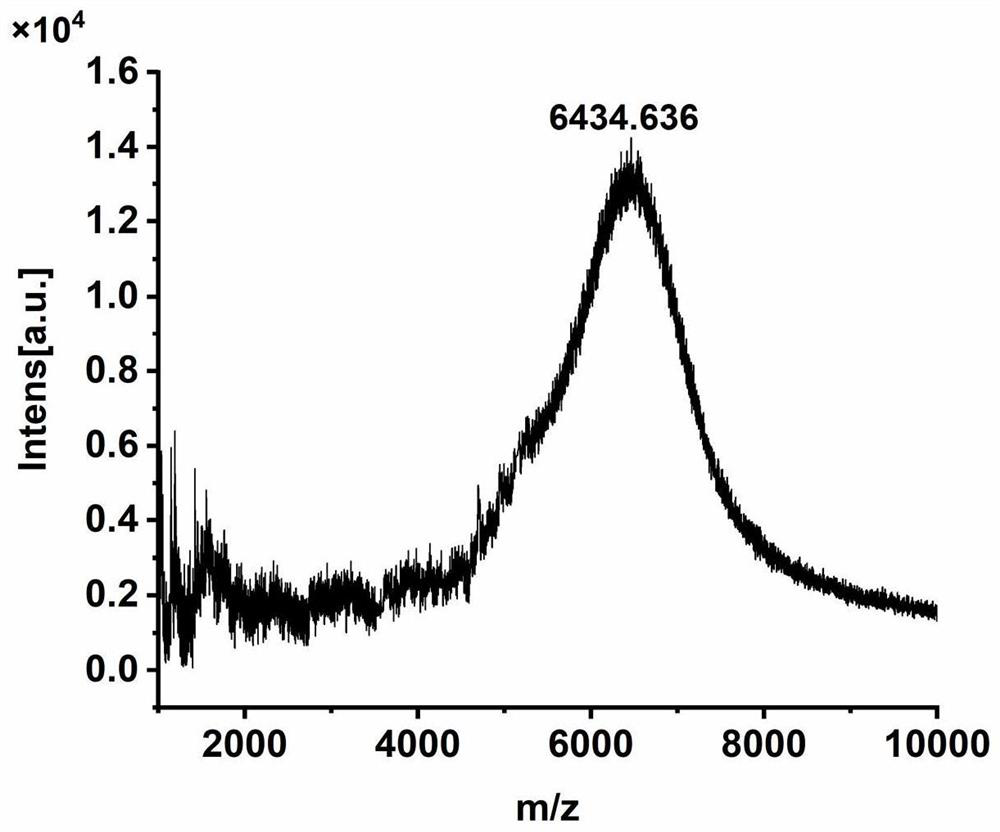
[0051] Example 3: Preparation of FB-Dox-Suc-KIGLFRWR-NPs
[0052] 1. Materials
[0053] 2,3-Dimethylmaleic anhydride (DMMA, 99%), ε-polylysine (ε-PL, Mw: 3000-5000), triethylamine, N-hydroxysuccinimide (NHS, 99 %), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI, 99%); ordinary dialysis bag (molecular weight 2000D), pure water, glacial acetic acid.
[0054] 2. Preparation method
[0055] (1) Synthesis of β-carboxyamide polylysine (FB):
[0056] Add 180 mg of DMMA to 15 mL of ε-polylysine aqueous solution dissolved in 160 mg, add triethylamine to adjust the pH to be alkaline, add 190 mg of NHS after stirring and dissolving, add 240 mg of EDCI after dissolving, and stir and add triethylamine , keep the pH value of the reaction system at 8.5-9, and react overnight. The reaction solution was transferred into the activated dialysis bag (molecular weight 2000D), pure water was used as the dialysis medium, the volume ratio was 1:1000, and the dialysis medium was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com