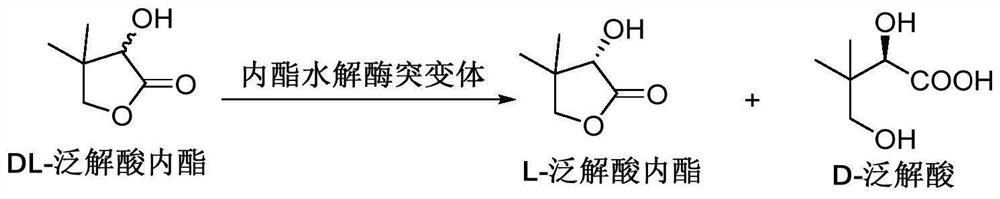
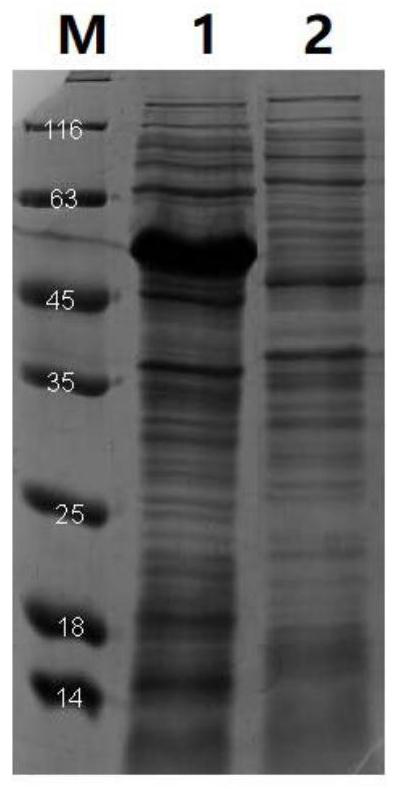
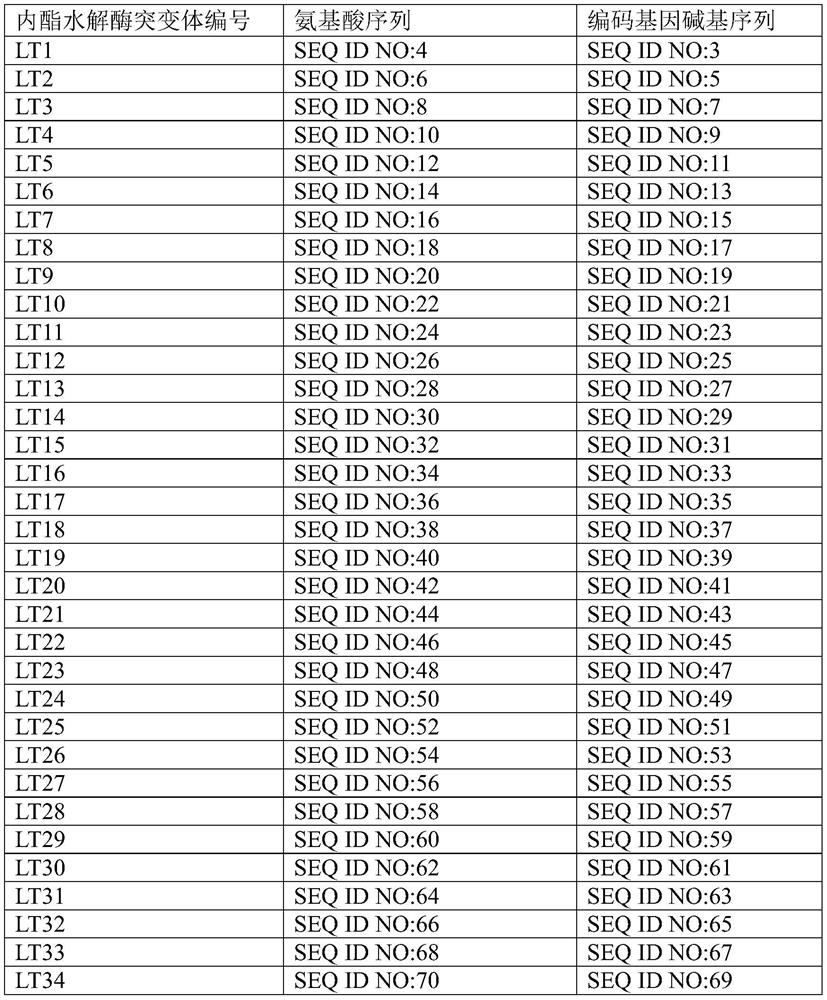
Lactone hydrolase mutant and application thereof
A technology of mutants and hydrolytic enzymes, applied in the fields of genetic engineering technology and biocatalysis, can solve problems such as low activity, poor stability, and difficulties in immobilizing cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1: Construction of wild-type lactone hydrolase expressing strain
[0081] For the derived lactone hydrolase LTO, according to the reported coding gene acid sequence SEQ ID NO: 1, Wuhan Jinkairui Bioengineering Co., Ltd. was entrusted to carry out the synthesis of the whole gene sequence, subcloned into the pGM-T vector, and transformed into E. coli E. .coli DH5α host to obtain the recombinant plasmid pGM-T-LT0 transformant containing the lactone hydrolase gene.
[0082] Design cloning primers:
[0083] Forward primer F1: 5'- CATATG CCTTCCACCATTTCTG-3’
[0084] Forward primer R1: 5'- CTCGAG CTAGTCATACAACTTG-3’
[0085] The pGM-T-LT0 plasmid was extracted as the gene template, and the gene encoding LTO was amplified by PCR with F1 and R1 forward and reverse primers, and the amplified fragment was about 1.2 kb. 50 μl PCR reaction system: 1 μl each of primers F1 and R1, 3 μl plasmid template, 25 μl 2×Premix PrimeSTAR HS, 10 μl sterilized distilled water. PCR...
Embodiment 2
[0093] Example 2: Obtaining mutants of lactone hydrolase with excellent performance by directed evolution
[0094] Design mutation primers:
[0095] Forward primer F2: 5'-TAATACGACTCACTATAGGG-3'
[0096] Reverse primer R2: 5'-TCGCTTGGAACATTATTAAC-3'
[0097] Using the pET-28-LTO plasmid containing the gene sequence of SEQ ID NO: 1 as a template, and using F2 and R2 as forward and reverse primers, a random mutation library was constructed by error-prone PCR technology. Error-prone PCR system 50 μl: 25 μl 2×TaqPCR MaterMix, 1 μl each of primers F2 and R2, 1 μl pET-28-LT0 plasmid, 0.5 μl dGTP (10 mM), MnCl 2 (10mM) 1 μl, sterile water 20.5 μl. PCR conditions: 95°C for 5 min; 30 cycles of 95°C for 30 s, 55°C for 30 s, 72°C for 1.5 min; last at 72°C for 10 min.
[0098] The PCR product was detected by agarose gel electrophoresis, and the amplified product was about 1200bp. The error-prone PCR gene products were recovered by a gel recovery kit, and used as large primers for who...
Embodiment 3
[0108] Example 3: Construction of yeast engineering bacteria and secretion and expression of lactonohydrolase mutants
[0109] Using pPICZαA as the shuttle vector and Pichia pastoris as the expression host GS115, a yeast engineering bacterium producing lactone hydrolase mutant was constructed to realize the extracellular secretory expression of the lactone hydrolase mutant.
[0110] Design cloning primers:
[0111] Forward primer F3: 5'- GAATT CGCCTTCCACCATTTCTG-3’
[0112] Forward primer R3: 5'- GCGGCCGC CTAGTCATACAACTTG-3’
[0113] The recombinant plasmid contained in the lactone hydrolase mutant strain TB4 obtained in Example 2 is pET-28-LT-23, and the lactone hydrolase mutant amino acid sequence of its expression is SEQ ID NO: 48, and the corresponding gene sequence is SEQ ID NO: 47.
[0114] The plasmid pET-28-LT-23 of the mutant strain strain TB4 was extracted by a plasmid extraction kit, and the plasmid pET-28-LT-23 of the mutant strain strain TB4 was taken as th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com