Preparation method of flurbiprofen
A technology of flurbiprofen and o-fluoroaniline, which is applied in the field of preparation of flurbiprofen, can solve the problems of many by-products, unsatisfactory amplification, harsh reaction conditions, etc., achieve high yield and purity of intermediate products, and improve overall Safe operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
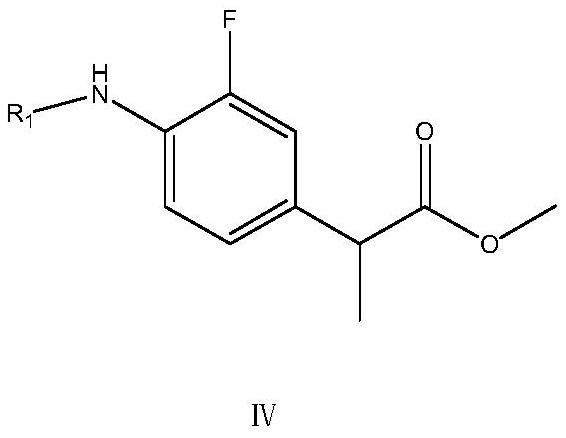
[0049] The preparation method of this flurbiprofen comprises the following steps:
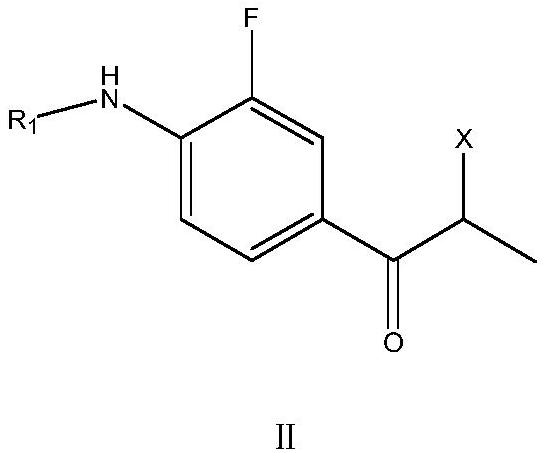
[0050] Under the action of Lewis acid, the acylation reaction of o-fluoroaniline or N-substituted o-fluoroaniline and 2-halopropionyl halide is preferably carried out in a water-insoluble organic solvent to obtain the corresponding intermediate compound of formula II;
[0051]
[0052] In the above formula II, R 1 Selected from acetyl, propionyl, H or Boc group; said X is the corresponding halogen in 2-halopropionyl halide; the halogen here can be chlorine, bromine or iodine, and the halogen of two corresponding halogen substitution positions They can be the same or different, preferably they are all chlorine, preferably chloropropionyl chloride. The above-mentioned water-insoluble organic solvent is preferably selected from one or more of cyclohexane, petroleum ether, n-heptane, methylene chloride and ethylene dichloride, preferably o-fluoroaniline or N-substituted o-fluoroaniline The mol...
Embodiment 1
[0077] Preparation of the first step intermediate formula II compound
[0078]
[0079] In a clean reactor, disperse 168g of aluminum trichloride (1.26mol) in 400mL of dichloromethane solvent, stir until dissolved, under the protection of nitrogen, lower the temperature to 10-15°C, and dropwise add 80g of 2 - Chloropropionyl chloride (0.63mol), control the temperature and carry out heat preservation and stirring for 1h, then, to the reaction solution of the above reaction system, slowly dropwise add 200mL of dichloromethylene chloride prepared in advance with 92g o-fluoroacetanilide (0.6mol) Methane clarifies the solution. After the dropwise addition, heat up to reflux and stir for 18-20h. After the reaction is over, cool down the reaction system by 0-5°C, and then add 300mL of 5% dilute hydrochloric acid solution dropwise. After the dropwise addition, fully stir 1h, standing for stratification, collecting the organic phase, and extracting the water phase once with dichloro...
Embodiment 2
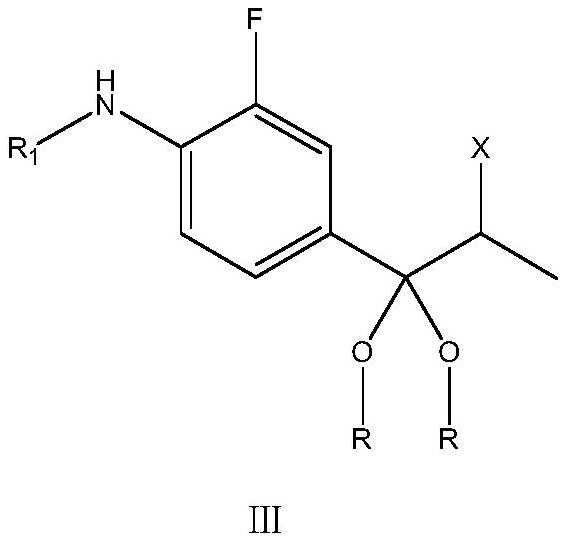
[0082]
[0083] 98g (0.4mol) of the formula II compound (intermediate 2) obtained according to the above-mentioned method was dissolved in 500mL of anhydrous methanol solvent, and then 53g of trimethyl orthoformate (0.5mol) and p-toluenesulfonic acid (0.02mol) were added, Then, the temperature was raised to 50-60° C. for 2 h. After the reaction is over, carry out vacuum distillation to recover methanol and trimethyl orthoformate, distill until no liquid flows out, then add 600mL of dichloromethane and 200mL of water to the solid concentrate, stir well, let stand to separate and collect After the organic phase was distilled under normal pressure to recover the solvent, 200 mL of isopropanol was added to the residue for sufficient beating treatment, and the filter cake was obtained by filtration. The filter cake was rinsed with a little isopropanol, and the obtained wet product was placed in a 50°C refrigerator. Drying under vacuum condition for 5-6h gave 99g of intermediate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com