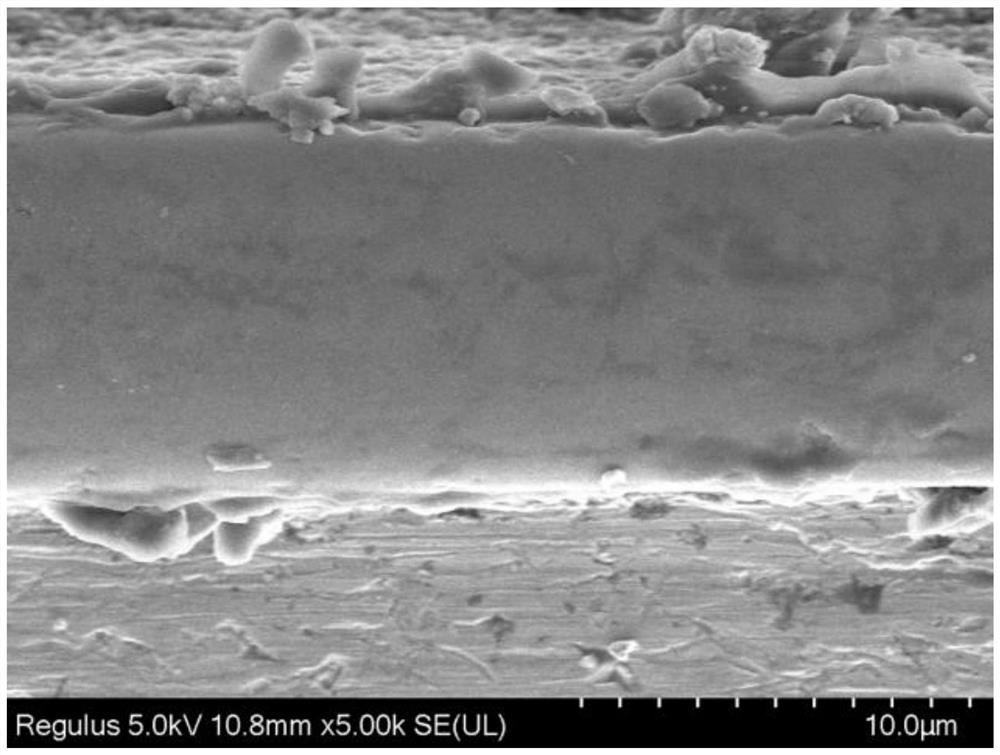
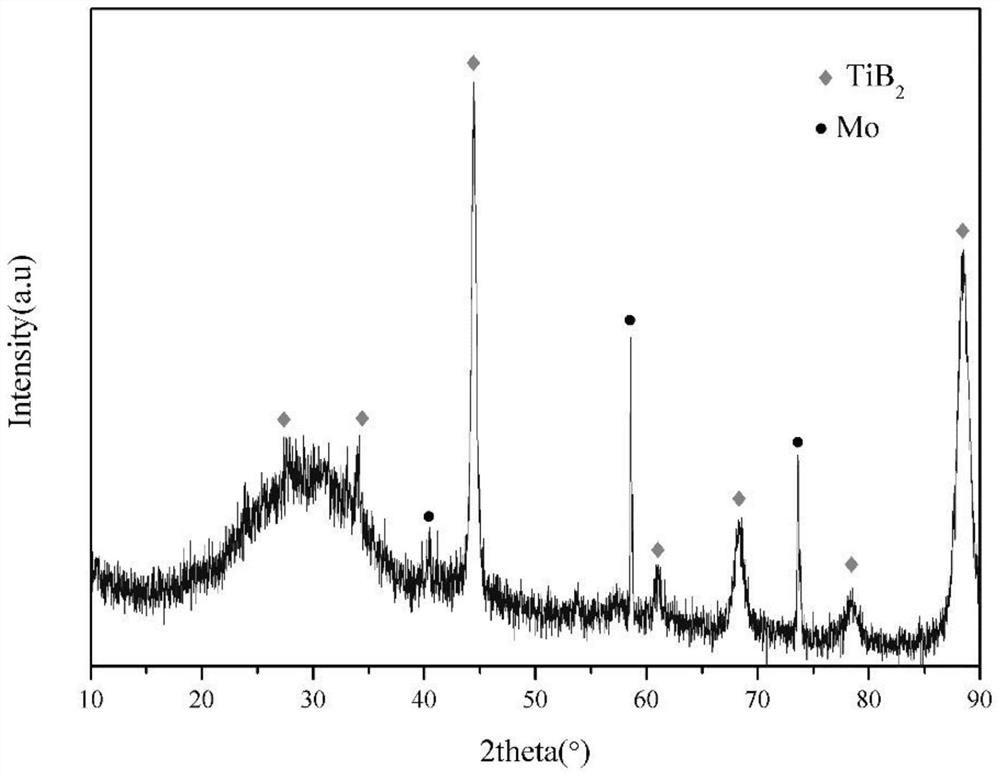
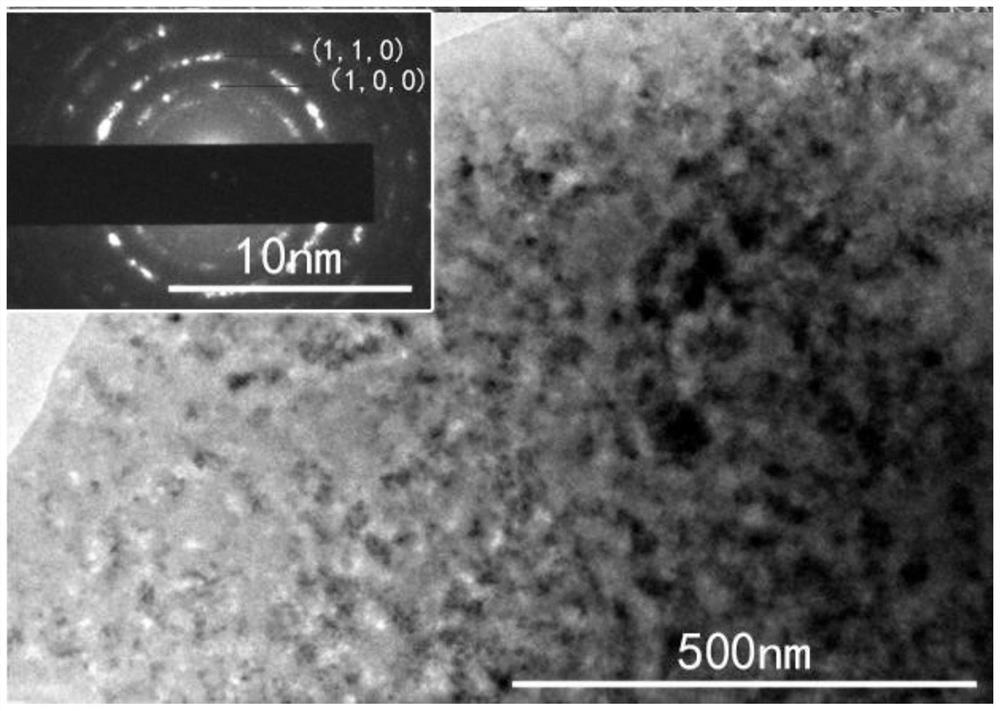
Metal boride coating and preparation method thereof
A metal boride and coating technology, which is applied in the field of metal boride coating and its preparation, can solve the problems of high brittleness, high cost, and poor bonding force of the coating, and achieve improved sintering density, low equipment cost, and improved binding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of the metal boride coating of the present invention comprises the following steps:
[0027] S1, preparing a solid mixed salt containing metal boride nanoparticles;
[0028] Mixing a certain proportion of solid inorganic salts, ball milling to micron level, adding metal boride nanoparticles to the ball milled mixed salt, the amount of metal boride nanoparticles added is 30% to 50% of the total weight of the mixed salt, Then add acetone liquid and ultrasonically disperse for 1-2.5 hours, vacuumize and heat in a vacuum drying oven, the heating temperature is 70°C-180°C, the vacuum degree is 30-150 Pa, and after vacuum heating treatment time is 0.5-2.5 hours, you can get Solid mixed salts containing metal boride nanoparticles.
[0029] S2, preparation of metal boride coating by electrophoretic deposition in nano-inorganic molten salt;
[0030] Put a certain proportion of solid inorganic salt into the crucible, heat to melt in an inert gas-protected...
Embodiment 1
[0038] NaF and AlF in a molar ratio of 3:2 3 Mix well, put into planetary ball mill and ball mill to micron level. In ball-milled NaF and AlF3 mixed salt, add zirconium diboride with an average particle diameter of 70 nanometers, and the weight of nano zirconium diboride is NaF and AlF 3 35% of the total weight of the mixed salt, then add acetone liquid and ultrasonically disperse for 1.5 hours, then put it into a vacuum oven for heating, the heating temperature is 150 ° C, and the vacuum degree is 100 Pa. After 1.8 hours of vacuum heat treatment, the acetone is completely volatilized to obtain di Solid-state NaF and AlF with uniform distribution of zirconium boride nanoparticles a Mixed salt; NaF and AlF in a molar ratio of 3:2 3 Inorganic salts were mixed and added to the graphite crucible, and under the protection of high-purity argon, they were melted in a resistance furnace at a temperature of 950°C. After complete melting, the zirconium diboride nanoparticles NaF and A...
Embodiment 2
[0040]Thoroughly mix NaCl and KCl with a molar ratio of 1:1, and put them into a planetary ball mill for ball milling to micron level. Add titanium diboride with an average particle size of 50 nanometers in the ball-milled NaCl and KCl mixed salt, the weight of nano-titanium diboride is 45% of the total weight of NaCl and KCl mixed salt, then add acetone liquid and ultrasonically disperse for 2 hours, and then placed in a vacuum oven for heating at a temperature of 130°C and a vacuum of 120 Pa. After 1.5 hours of vacuum heat treatment, the acetone was completely volatilized to obtain a solid NaCl and KCl mixed salt with titanium diboride nanoparticles evenly distributed. NaCl, KCl and AlCl with a molar ratio of 17:17:66 3 Inorganic salts were mixed and added to a quartz crucible, and under the protection of high-purity argon, they were melted in a resistance furnace at a temperature of 710°C. After complete melting, the NaCl and KCl containing titanium diboride nanoparticles p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com