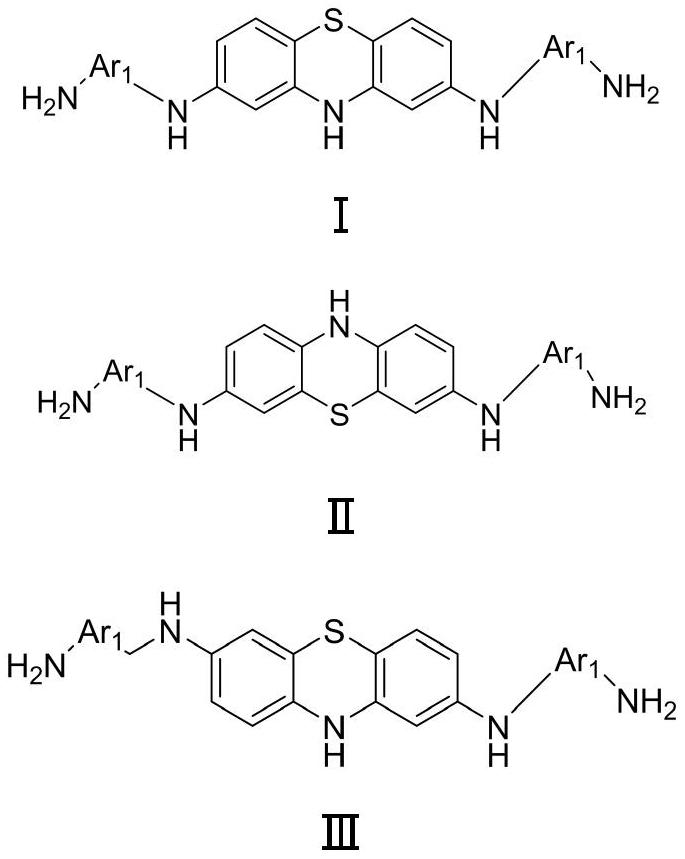
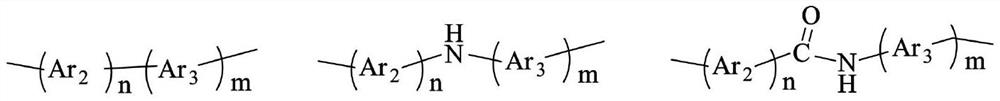Diamine containing phenothiazine structure and preparation method for synthesizing polyimide
A technology of polyimide and phenothiazine, which is applied in the field of material science, can solve the problems of limited barrier performance improvement, easy breakage and falling off, and cannot meet high requirements, and achieve high electron density, good rigid structure, rich and diverse functions sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] This example provides N 1 ,N 1 Synthesis of '-(10H-phenothiazine-2,8-diyl)bis(benzene-1,4-diamine):
[0043]
[0044] S1. Synthesis of intermediate 10H-phenothiazine-2,8-diamine:
[0045] Add 3.57g (0.01mol) of 2,8-dibromo-10H-phenothiazine, an appropriate amount of cuprous oxide, 50ml of NMP, and 13ml of ammonia water (29%, 0.2mol) into a 200ml pressure bottle, under argon protection, and react at 100°C. After the reaction was completed, the reaction solution was poured into ice water, extracted with dichloromethane, and the solvent was removed under reduced pressure. The product was purified by column chromatography using dichloromethane:n-hexane=2:1 (volume ratio) as the mobile phase and silica gel as the stationary phase. , the product was collected and spin-dried, and dried in vacuum at 80°C for 24h to obtain an intermediate. The intermediate structure is as follows:
[0046]
[0047] S2. Synthesis of intermediate N 2 ,N 8 -bis(4-nitrophenyl)-10H-phenothi...
Embodiment 2
[0053] This example provides
[0054] N 2 -(5-aminopyridin-2-yl)-N 7 -Synthesis of (6-aminopyridin-3-yl)-10H-phenothiazine-2,7-diamine:
[0055]
[0056] S1. Synthesis of intermediate 10H-phenothiazine-2,7-diamine:
[0057] Add 3.57g (0.01mol) of 2,7-dibromo-10H-phenothiazine, an appropriate amount of cuprous oxide, 50ml of NMP, and 13ml of ammonia water (29%, 0.2mol) into a 200ml pressure bottle, under argon protection, and react at 100°C. After the reaction was completed, the reaction solution was poured into ice water, extracted with dichloromethane, and the solvent was removed under reduced pressure. The product was purified by column chromatography using dichloromethane:n-hexane=2:1 (volume ratio) as the mobile phase and silica gel as the stationary phase. , the product was collected and spin-dried, and dried in vacuum at 80°C for 24h to obtain an intermediate. The intermediate structure is as follows:
[0058]
[0059] S2. Synthetic intermediates
[0060] N ...
Embodiment 3
[0068] This example provides N 1 ,N 1 Synthesis of '-(dibenzo[b,d]furan-2,8-diyl)bis(benzene-1,3-diamine):
[0069]
[0070] S1. Synthesis of intermediate 10H-phenothiazine-3,7-diamine:
[0071] Add 3.57g (0.01mol) of 3,7-dibromo-10H-phenothiazine, an appropriate amount of cuprous oxide, 50ml of NMP, and 13ml of ammonia water (29%, 0.2mol) into a 200ml pressure bottle, under argon protection, and react at 100°C. After the reaction was completed, the reaction solution was poured into ice water, extracted with dichloromethane, and the solvent was removed under reduced pressure. The product was purified by column chromatography using dichloromethane:n-hexane=2:1 (volume ratio) as the mobile phase and silica gel as the stationary phase. , the product was collected and spin-dried, and dried in vacuum at 80°C for 24h to obtain an intermediate. The intermediate structure is as follows:
[0072]
[0073] S2. Synthesis of intermediate N 3 ,N 7 -bis(3-nitrophenyl)-10H-phenot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com