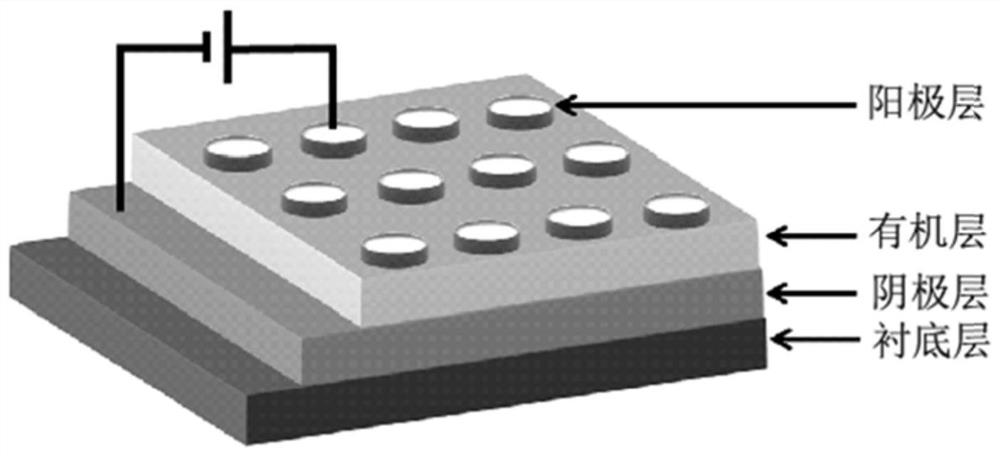
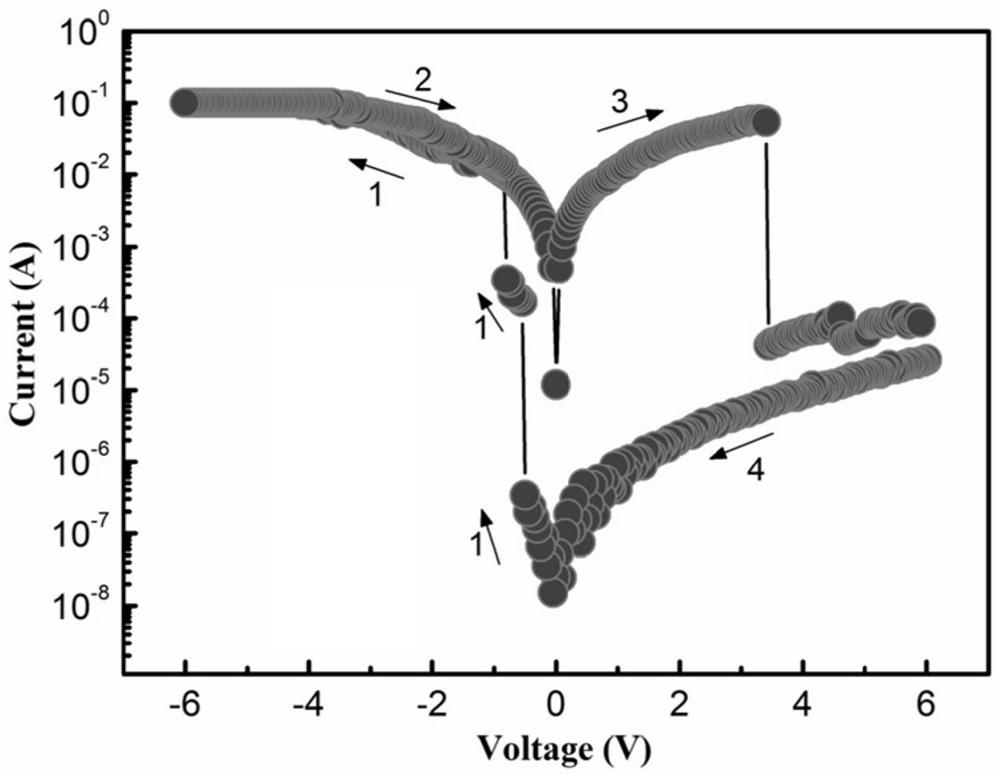
Triphenylamine-carbazole-naphthylbenzimidazole terpolymer, preparation method and application of terpolymer in electric storage device
A naphthalene benzimidazole terpolymer and terpolymer technology, which is applied in the fields of terpolymer and its synthesis, electrical storage devices and its preparation, can solve the problem of high raw material and manufacturing costs, complex synthesis methods, and few researches and other problems, to achieve the effect of easy film formation, simple synthesis method, and low misreading rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The preparation method of described triphenylamine-carbazole-naphthalene benzimidazole terpolymer comprises the following steps:
[0051] Step 1, synthesizing monomers containing naphthalene halogenated benzimidazoles.
[0052] The naphthalene-containing halogenated benzimidazole monomer is a halogenated benzimidazolobenzisoquinolinone monomer, and the 4-position and 7-position of the benzimidazole introduce a halogenated group X 1. The structural formula of the described naphthalene-containing halogenated benzimidazole monomer is:
[0053]
[0054] Among them, the halo group X 1 selected from chlorine, bromine, iodine, preferably bromine or iodine; R 3 and R 4 Each is independently selected from hydrogen, alkyl or alkoxy, preferably selected from hydrogen, alkyl containing 1-3 carbon atoms or alkoxy containing 1-3 carbon atoms, more preferably hydrogen. In a preferred mode of the present invention, 9,12-dibromo-7H-benzoimidazo[2,1-a]benzo[de]isoquinolin-7-one i...
Embodiment 1
[0144] in N 2 Under the atmosphere, put 0.1329g of 3,6-dibromo-1,2-phenylenediamine, 0.0965g of 1,8-naphthalene dicarboxylic anhydride, and 10mL of glacial acetic acid in a three-necked flask, and magnetically stir at 110°C for 6 hours. , cool the reaction to room temperature, filter the crude product after precipitation, wash with deionized water until neutral, put the obtained crude product in a vacuum drying oven for drying, the temperature of vacuum drying is 80-85 ° C, the time of vacuum drying 12h, vacuum drying pressure is -30 ~ -29kPa. The dried crude product was separated and purified by column chromatography to obtain 9,12-dibromo-7H-benzoimidazo(2,1-a)benzo(de)isoquinolin-7-one monomer.
Embodiment 2
[0146] Prepare a clean 100 mL Schlenk bottle, mix 1.70 g of 4-methoxyaniline, 8.08 g of 1-bromo-4-iodobenzene, 0.12 g of tris(dibenzylideneacetone) dipalladium, 0.30 g of 1,1 '-Bis(diphenylphosphino)ferrocene (DPPF), 3.92g of sodium tert-butoxide and 25mL of purified toluene were added to the bottle in turn. After the drug is dissolved, vacuumize and fill with nitrogen, repeat three times and then continue to pass nitrogen to keep the reaction in an anaerobic state. Turn on constant-temperature magnetic stirring, and slowly raise the temperature to 110° C. for 6 h. As the reaction proceeded, the solution was brown, and a gray solid appeared on the wall of the bottle 1 h after the reaction started. After 6 hours, the reaction was completed and the solution in the bottle was separated. The upper layer was a tan organic phase, and the lower layer was a gray mud.
[0147] The mixed solution was rotary evaporated to remove toluene, the mixture was dissolved in dichloromethane and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com