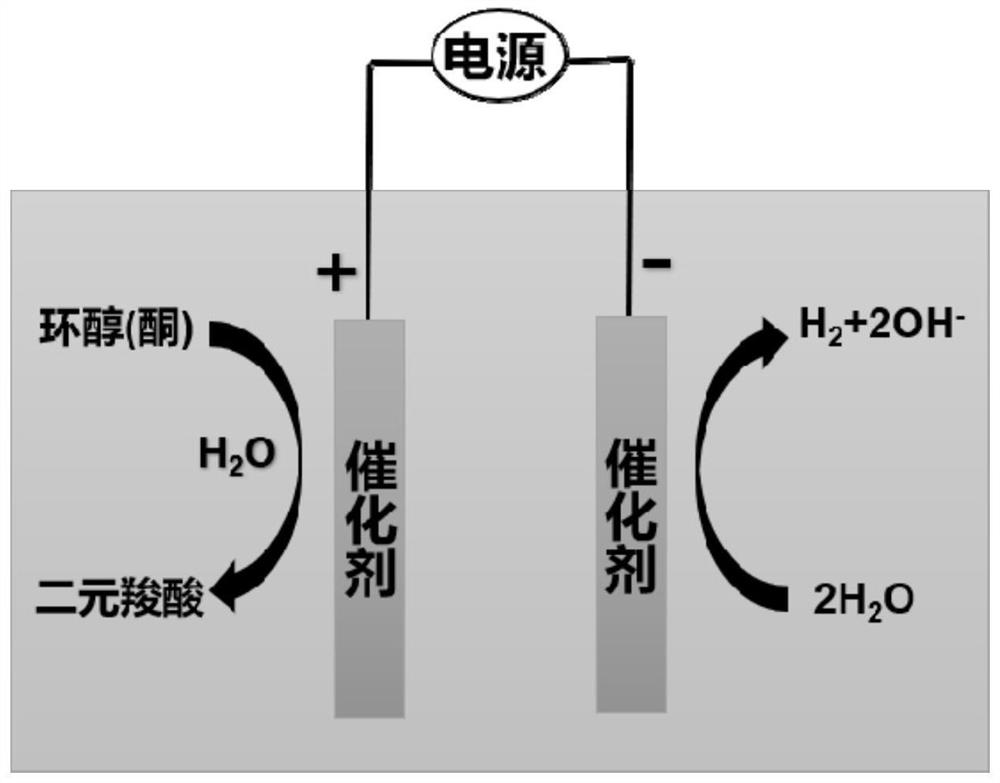
Method for producing hydrogen by coupling dicarboxylic acid prepared by electrocatalytic oxidation of cyclic alcohol/cyclic ketone
An electrocatalytic oxidation and dicarboxylic acid technology, which is applied in the direction of catalyst activation/preparation, chemical instruments and methods, electrolytic organic production, etc., can solve problems such as environmental pollution, corrosive reaction equipment, and non-compliance with green chemistry, and achieve product The effect of high yield and broad application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
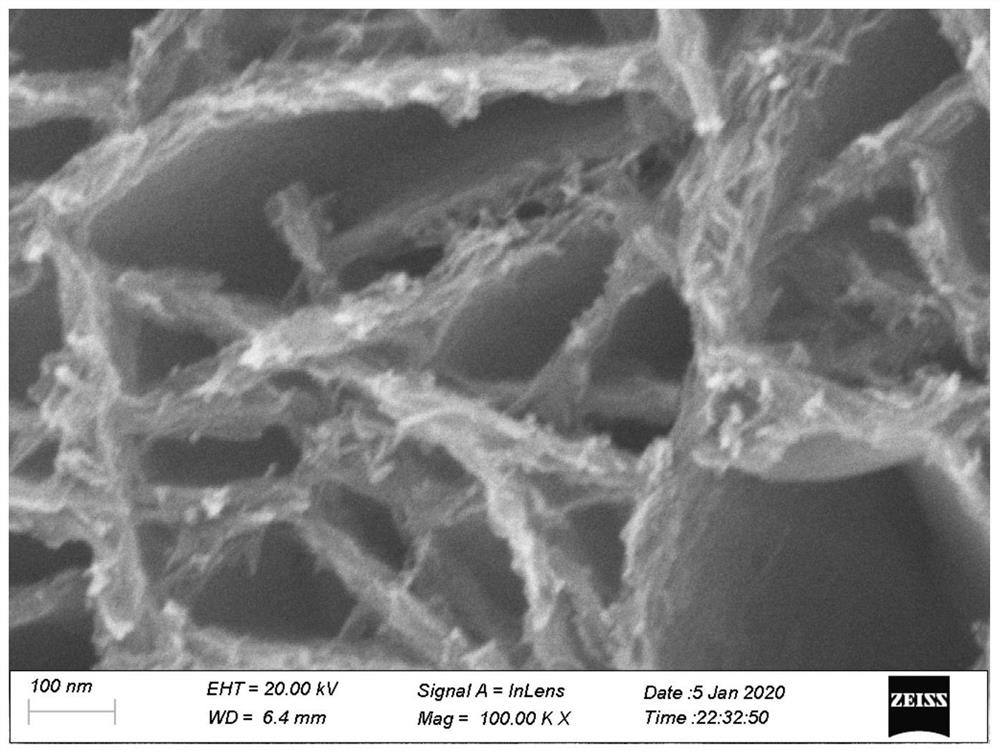
[0033] The carbon cloth was sonicated in analytically pure absolute ethanol, analytically pure acetone, and deionized water for 15 minutes to remove surface impurities. Prepare 25mL of an aqueous solution containing 1.5g of urea, 0.9g of ammonium tetrafluoride and 0.5g of nickel nitrate as a hydrothermal reaction solution, soak the carbon cloth in the prepared hydrothermal reaction solution, and react at a constant temperature of 110°C for 8 hours, removed, washed with deionized water and dried to obtain the precursor material. The prepared precursor material was put into a quartz boat together with 100 mg of sodium hypophosphite and transferred into a tube furnace. Phosphating at 300° C. for 2 hours under an argon atmosphere to obtain a porous network nickel phosphide nano-electrocatalyst. The scanning electron microscope image of the porous network nickel phosphide nano-electrocatalyst is shown in figure 2 middle.
Embodiment 2
[0036] Sonicate the nickel foam in analytically pure absolute ethanol, analytically pure acetone, and deionized water for 15 minutes to remove surface impurities. Prepare 25mL of the urea that comprises 1.5g, the ammonium tetrafluoride of 0.9g, the cobalt nitrate of 0.5g and the aqueous solution of the nickel nitrate of 0.5g as hydrothermal reaction solution, nickel foam is soaked in the hydrothermal reaction solution of configuration, in React at a constant temperature of 120° C. for 8 hours, take it out, wash with deionized water and dry to obtain a precursor material. The prepared precursor was put into a quartz boat together with 100 mg of potassium hypophosphite and transferred into a tube furnace. Phosphating at 300° C. for 2 hours under an argon atmosphere to obtain cobalt-nickel phosphide nano-electrocatalysts.
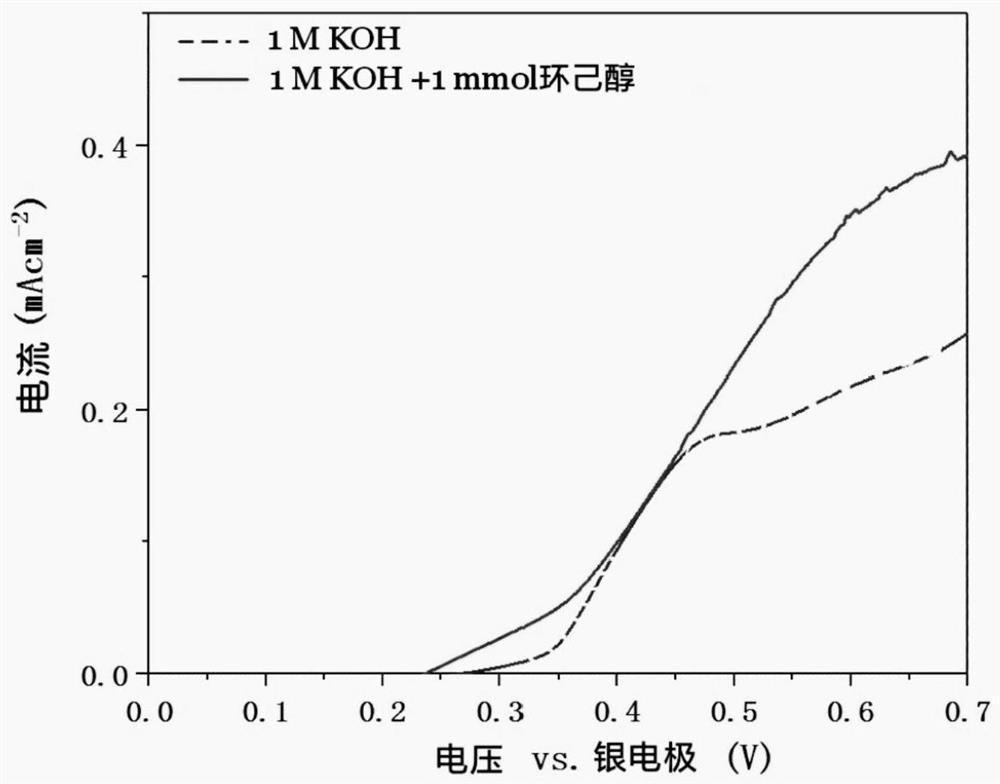
[0037]Prepare 20 mL of 1 M potassium hydroxide solution containing 1 mg of cyclohexanone as the electrolyte, use the cobalt-nickel phosphide nano-electrocata...
Embodiment 3
[0039] The nickel sheet was sonicated in analytically pure absolute ethanol, analytically pure acetone, and deionized water for 15 minutes to remove surface impurities. Prepare 25mL of an aqueous solution containing 1.5g of hexamethylenetetramine, 0.9g of ammonium tetrafluoride, 0.5g of cobalt nitrate and 0.2g of manganese nitrate as a hydrothermal reaction solution, and soak the nickel sheet in the configured hydrothermal reaction solution. The reaction liquid was reacted at a constant temperature of 120° C. for 8 hours, taken out, washed with deionized water and dried to obtain a precursor material. The prepared precursor material was put into a quartz boat together with 100mg of red phosphorus and transferred into a tube furnace. Phosphating at 350° C. for 2 hours under an argon atmosphere to obtain cobalt-manganese phosphide nano-electrocatalysts.
[0040] Prepare 20 mL of 1 M potassium hydroxide solution containing 1 mg of cyclopentanol as the electrolyte, use the cobalt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com