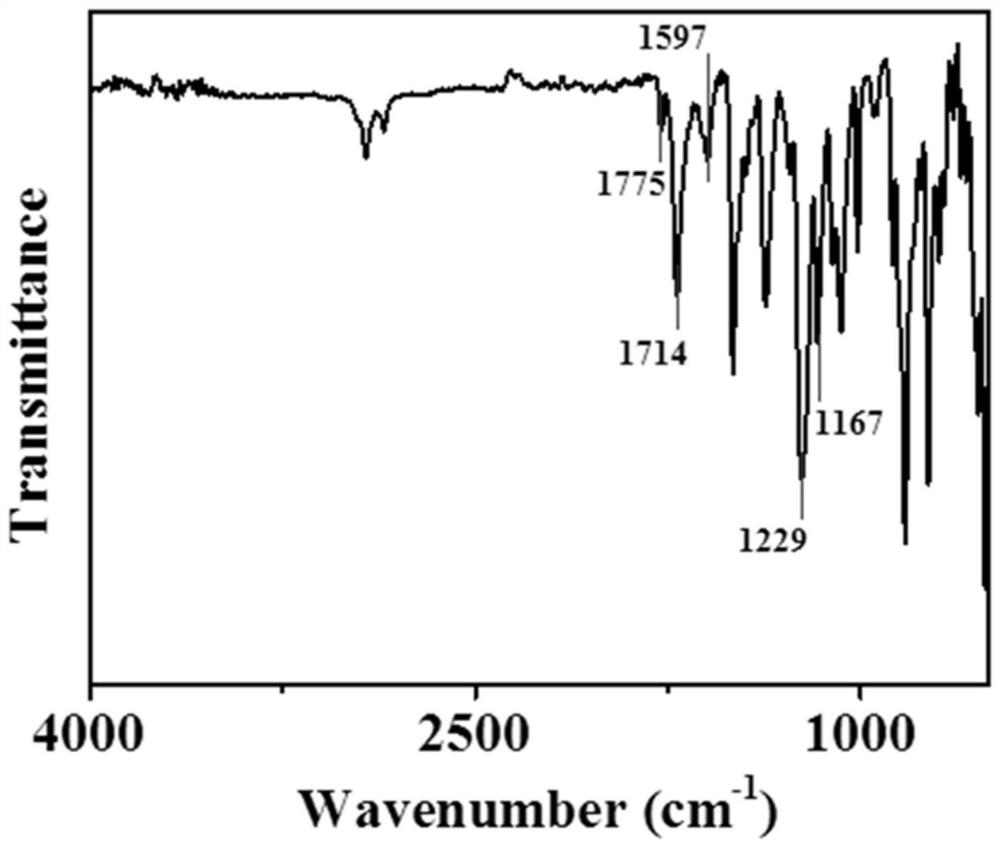
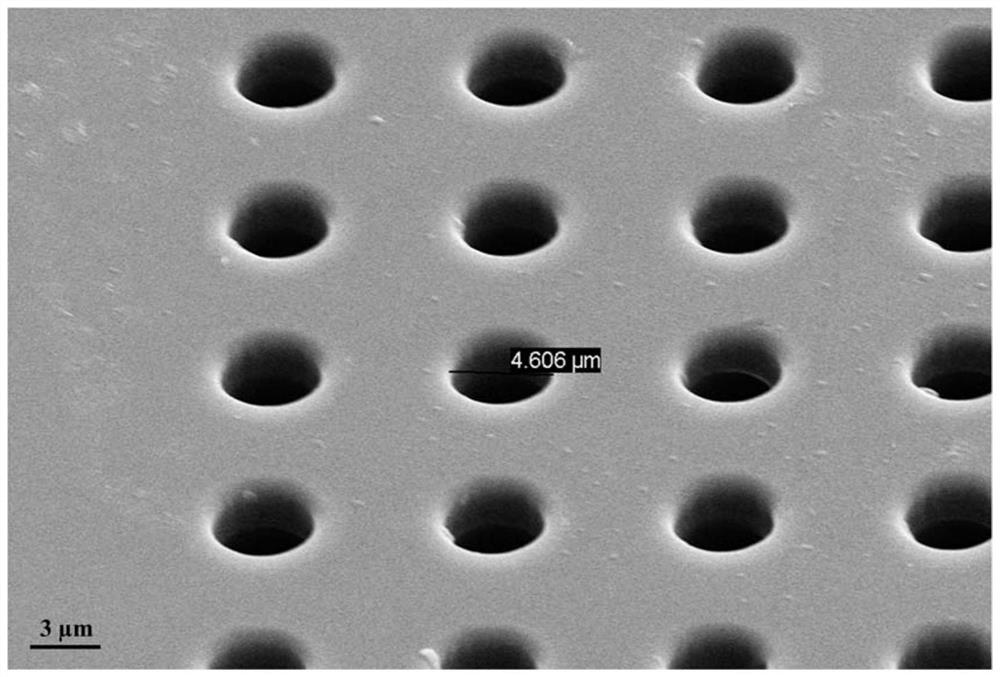
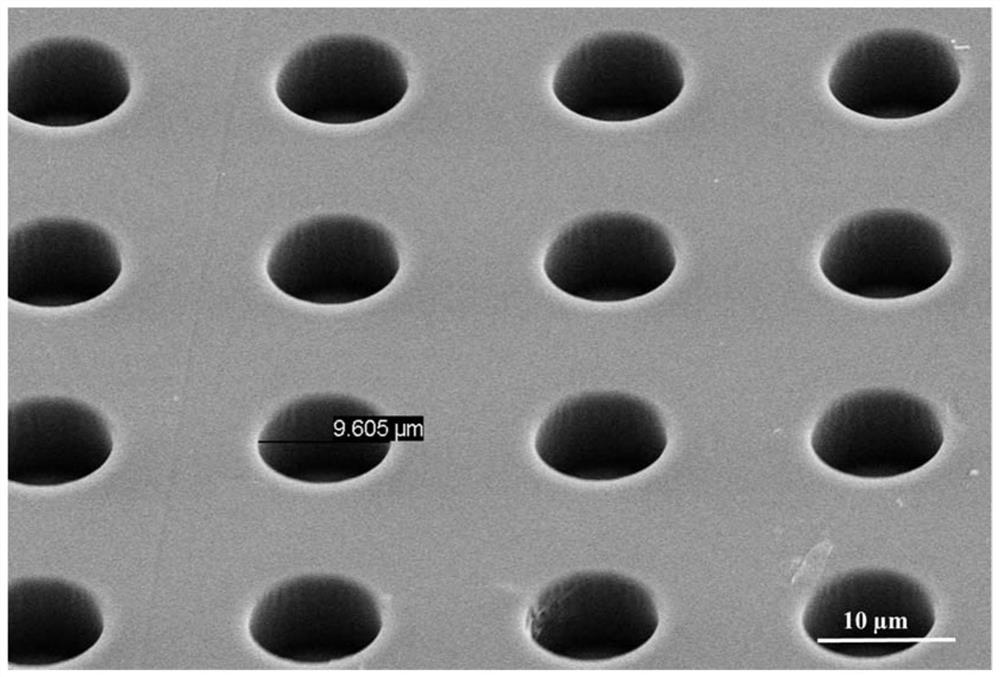
Photosensitive resin, photoresist and preparation method and application thereof
A technology of photosensitive resin and photoresist, which is used in optomechanical equipment, photosensitive materials for optomechanical equipment, optics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] One embodiment of the present invention also provides a method for preparing a photosensitive resin, including the following steps S10-S20.
[0066] Step S10: Add dianhydride monomer, diamine monomer and 3,3'-dicarboxy-4,4'-bis(4-amino-2-trifluoromethylphenoxy)biphenyl monomer in the first Mixing and dissolving in an organic solvent for condensation reaction to prepare polyamic acid prepolymer.
[0067] Further, the conditions of the condensation reaction are: react in an ice-bath environment for 8h-24h.
[0068] Furthermore, the condensation reaction is carried out under anhydrous and oxygen-free conditions.
[0069] Wherein, the structural formula of dianhydride monomer is:
[0070] The structural formula of diamine monomer is:
[0071] The structural formula of 3,3'-dicarboxy-4,4'-bis(4-amino-2-trifluoromethylphenoxy)biphenyl monomer is:
[0072] The structural formula of polyamic acid prepolymer is:
[0073]
[0074] In a specific example, the dianhydr...
Embodiment 1
[0125] Preparation of step 1, 3,3'-dicarboxy-4,4'-bis(4-amino-2-trifluoromethylphenoxy)biphenyl monomer:
[0126] (1) Add 5.48g (20mmol) 4,4'-dihydroxy-3,3'-biphenyldicarboxylic acid and 9.20g (44mmol) 2-fluoro-5-nitrobenzotrifluoride to 160ml N,N-diphenyl After dissolving in methylformamide (DMF), add 6.08g (44mmol) potassium carbonate (K 2 CO 3 ) as a catalyst, toluene as an azeotrope of water produced in the reaction process, reflux reaction for 20h under the protection of nitrogen to generate 3,3'-dicarboxy-4,4'-bis(4-nitro-2-trifluoroform 10.42g of phenyloxy)biphenyl. The chemical reaction formula is as follows:
[0127]
[0128] (2) 6.52g (10mmol) of 3,3'-dicarboxy-4,4'-bis(4-nitro-2-trifluoromethylphenoxy)biphenyl prepared in step (1) in the absence of Water is dissolved in 100ml tetrahydrofuran (THF) under anaerobic conditions, a catalytic amount of palladium carbon (Pd / C) is added, hydrogen (H 2 ) as a reducing agent and maintain a pressure of 5 bar for 4 hour...
Embodiment 2
[0140] Step 1, with the step 1 of embodiment 1.
[0141] Step 2, preparation of photosensitive resin:
[0142] (1) Under anhydrous and oxygen-free conditions, add 4.15 g (7 mmol) of 3,3'-dicarboxy-4,4'-di( 4-amino-2-trifluoromethylphenoxy)biphenyl monomer, 0.60 g (3 mmol) of 4,4'-diaminodiphenyl ether (ODA, diamine monomer) and 50 ml of DMF. After all substances in the system were completely dissolved, 3.22g (10mmol) of 3,3',4,4'-benzophenonetetracarboxylic dianhydride (BTDA, dianhydride monomer) was added, and stirred for 8h in an ice-bath environment.
[0143] (2) After the reaction in (1) is completed and the reaction system returns to 25°C, add 5.4ml of acetic anhydride and 1ml of pyridine to continue the reaction for 24 hours, and then purify by methanol precipitation, filtration, and Soxhlet extraction.
[0144] The chemical reaction formula of step 2 is as follows:
[0145]
[0146] Step 3, with step 3 of embodiment 1.
[0147] Step 4, with step 4 of embodiment 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com