Enzymatic synthesis of chiral amino alcohol compounds
A technology of amino acid and biocatalysis, which is applied in the preparation of amino hydroxyl compounds, the preparation of organic compounds, methods based on microorganisms, etc. It can solve the problems of low yield, many by-products, and the maximum can only reach 50%. The effect of high conversion rate, less by-products and good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Construction and induced expression of imine reductase genetically engineered bacteria
[0025] The imine reductase gene was synthesized by General Biosystems (Anhui) Co., Ltd., connected to the pET28a vector, and then transformed into Escherichia coli BL21 (DE3) competent cells, and single clones were picked to obtain recombinant bacteria. Select a single colony on the plate and inoculate it into 20mL LB medium containing the corresponding antibiotic, cultivate it for about 12 hours as a seed solution, inoculate it into 700mL LB medium containing the corresponding antibiotic according to the inoculation amount of 1%, and inoculate it on a shaker at 37°C and 200rpm Grow to OD 600 =0.6-0.8, add IPTG with a final concentration of 0.1 mmol / L and induce at 25°C for 12 hours. The bacterial cells were collected by centrifuging the culture medium at 6000rpm.
Embodiment 2
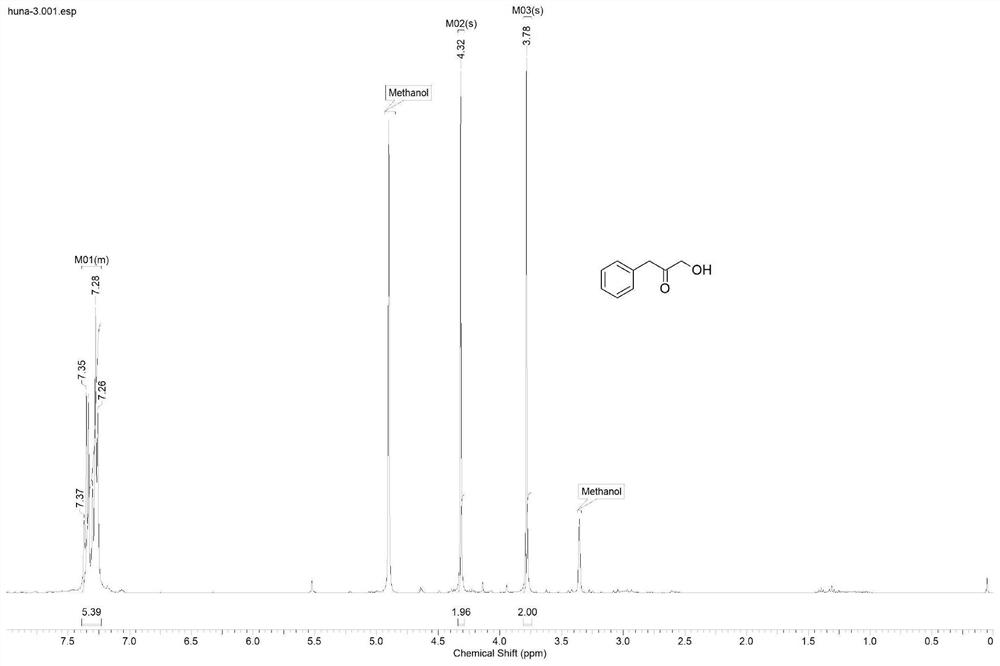
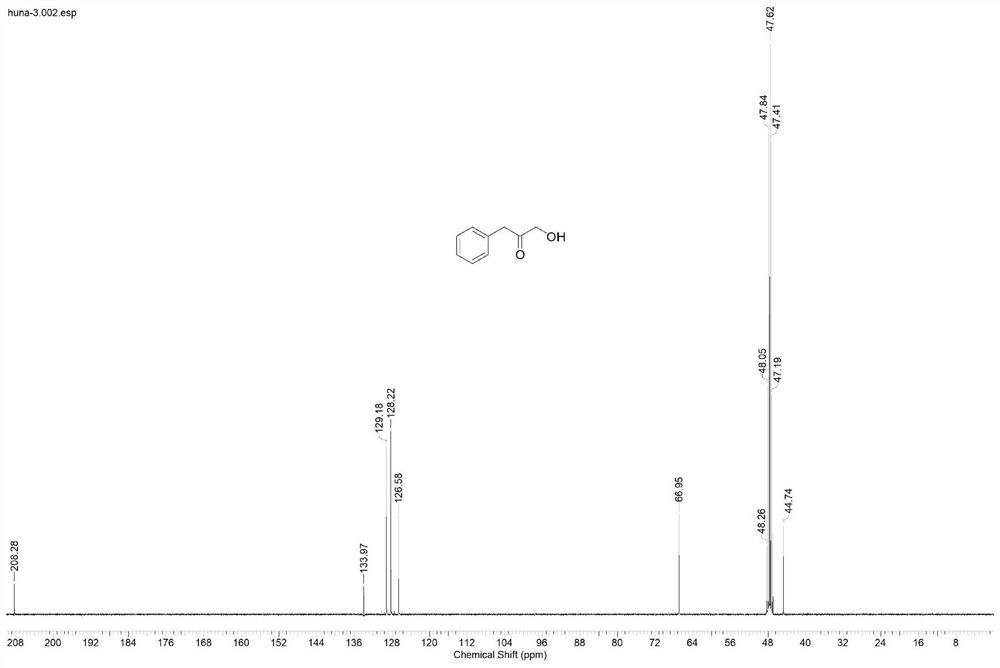
[0026] Example 2: Synthesis of α-hydroxyketone substrates
[0027] 200mL of absolute ethanol was used as a solvent, added to a 500mL flask, and 2.8mL of triethylamine (20mmol / L), 3.6g of paraformaldehyde (600mmol / L), 2.88g of n-butyraldehyde (200mmol / L) or 3.44g isovaleraldehyde (200mmol / L) or 4.8g phenylacetaldehyde (200mmol / L) or 5.36g phenylpropionaldehyde (200mmol / L) or 3.96g p-bromophenylacetaldehyde (100mmol / L) or 4.24g p-bromine Phenylpropionaldehyde (100mmol / L), under the catalysis of 2.88g thiazole salt (20mmol / L), heated and refluxed at 60°C for 96h, during the reaction, N 2 Protection, to obtain α-hydroxy ketone compound 1-hydroxy-2-pentanone or 1-hydroxy-4-methyl-2-pentanone or 1-hydroxy-3-phenyl-2-propanone or 1-hydroxy-4- Phenyl-2-butanone or 1-hydroxy-3-(4-bromophenyl)-2-propanone or 1-hydroxy-4-(4-bromophenyl)-2-butanone. The solvent in the system after the reaction is removed, the target compound is obtained through column chromatography separation, the targ...
Embodiment 3
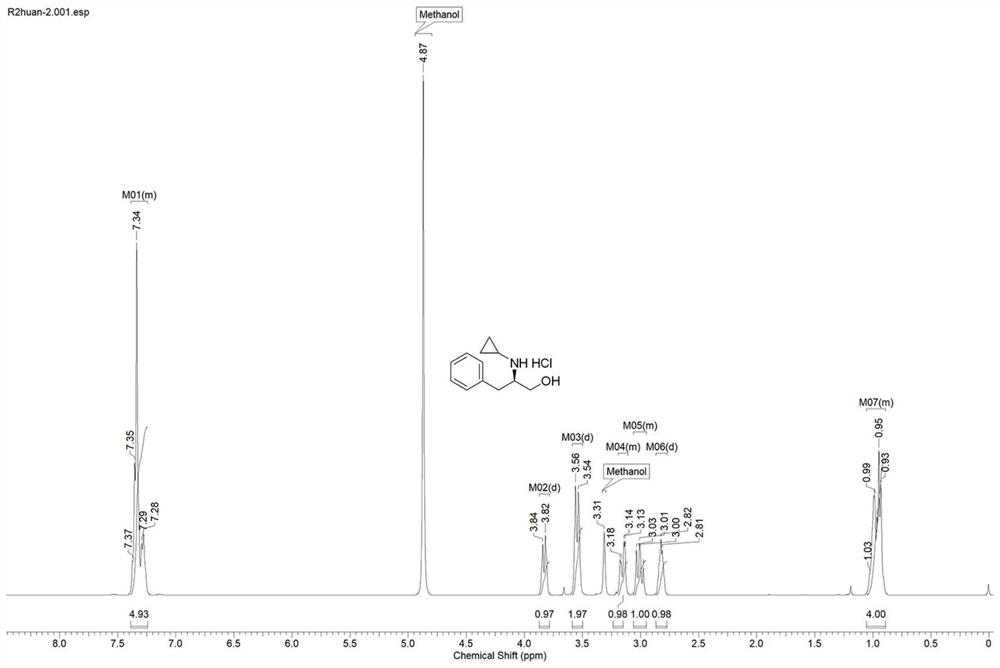
[0028] Example 3: Whole cells of imine reductase genetically engineered bacteria catalyze the reductive amination reaction of 1-hydroxy-3-phenyl-2-propanone and cyclopropylamine.
[0029]Weigh 0.05 g of imine reductase genetic engineering bacteria IR-27, IR-30, IR-36, IR-38, IR-40, IR-44, IR-51, IR-54, IR-56, IR -57, dissolved in 900 μL pH 8.0 K 2 HPO 4 -KH 2 PO 4 To the buffer, add 1.5 mg 1-hydroxy-3-phenyl-2-propanone (10 mmol / L) and dissolve it in 100 μL of anhydrous methanol, add 10 times equivalent of prepared cyclopropylamine (pH 8.0), 0.5 mg NADP + , 3U GDH enzyme powder, 7.2mg glucose (40mmol / L), after mixing evenly, react at 200rpm, 25℃ for 24h. After the reaction, the reaction system was treated with 1mol / L Na 2 CO 3 Adjust base and then extract with ethyl acetate, and detect by HPLC after the solvent is evaporated to dryness. The test results are: IR-27 catalyzes the reaction to obtain the S configuration product, the ee value is 99%, IR-36, IR-38, IR-40, IR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com