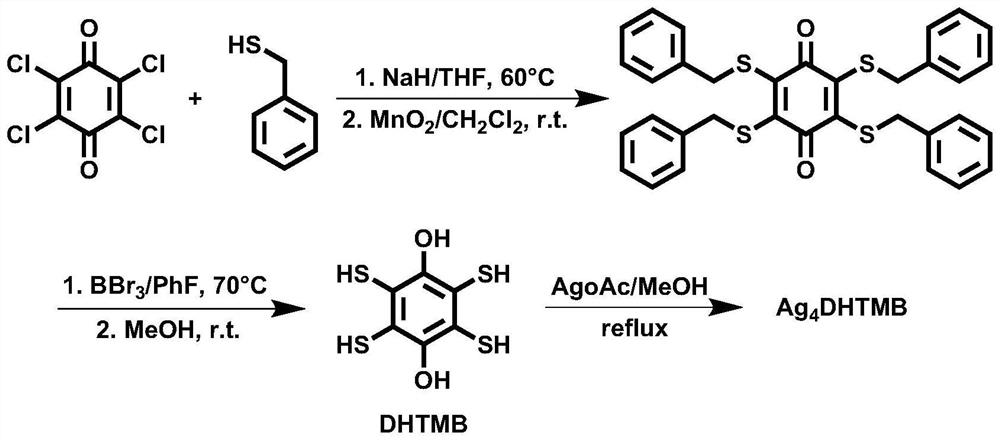
P-type thermoelectric material and preparation method thereof
A reaction and ligand technology, applied in the field of p-type thermoelectric materials and their preparation, can solve the problems of lack of raw material sources and complicated preparation methods, and achieve the effect of simple equipment and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1. Preparation of 1,4-dihydroxy-2,3,5,6-tetramercaptobenzene (DHTMB)
[0044] Flow chart such as figure 1 shown.
[0045] 1. Synthesis of 2,3,5,6-tetrabenzylthio-1,4-benzoquinone
[0046] 3.072g of sodium hydride was added to a 500mL two-necked bottle with a magnet, one bottle mouth was plugged with a rubber stopper, and the other bottle mouth was connected to a double-row tube. The gas in the bottle was withdrawn and argon was passed through, repeating 3 times. 300 mL of tetrahydrofuran was added, 9 mL of benzyl mercaptan was added while stirring, and 3.9 g of tetrachlorobenzoquinone was added after the reaction was performed at room temperature for 1 hour. The reaction system was heated to 60°C, and the temperature was lowered to room temperature after 12 hours of reaction. Water was added to the system, followed by extraction with dichloromethane three times, the extract was dried over anhydrous magnesium sulfate, and filtered to obtain a filtrate. The s...
Embodiment 2
[0051] Example 2. Preparation of Ag-DHTMB crystallites
[0052] Flow chart such as figure 1 shown.
[0053] 48mg DHTMB was added to a 100mL two-necked bottle with a magnet, one bottle mouth was plugged with a rubber stopper, and the other bottle mouth was connected to a double-row tube. The gas in the bottle was withdrawn and argon was passed through, repeating 3 times. 40 mL of degassed methanol and 116.8 mg of silver acetate were added sequentially (the molar ratio of DHTMB to silver acetate was 1:3.5). Heat to reflux and return to room temperature after 24 hours of reaction. The solid was obtained by filtration, washed with water, methanol and diethyl ether in turn, and dried in vacuum at 60°C to obtain Ag-DHTMB microcrystals.
Embodiment 3
[0054] Example 3. Characterization of DHTMB and Ag-DHTMB crystallites
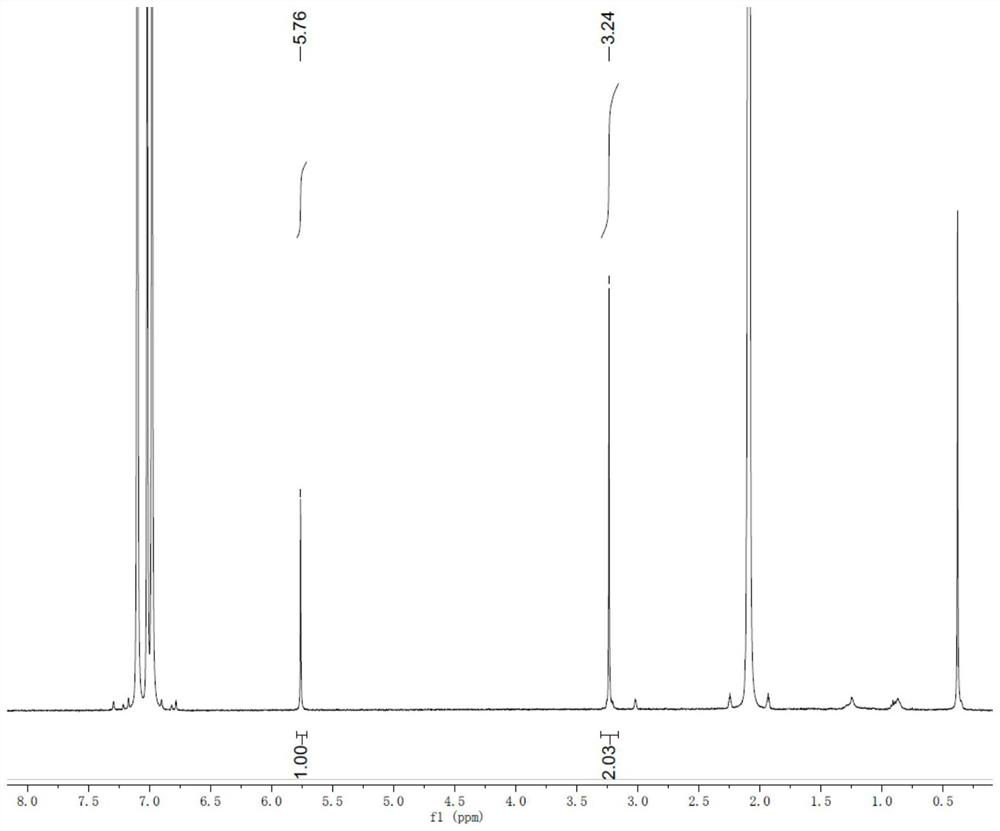
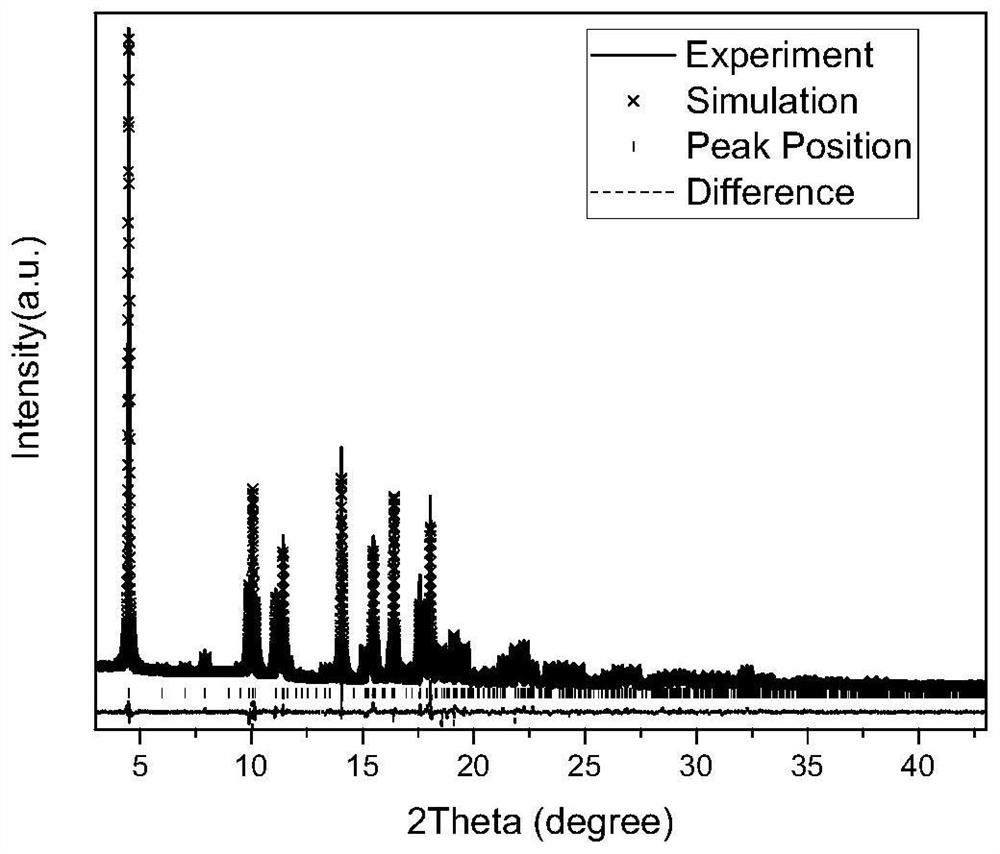
[0055]A small amount of prepared DHTMB was dissolved in deuterated toluene solvent and subjected to 1 H-NMR characterization; Ag-DHTMB crystallites were characterized by synchrotron radiation powder X-ray diffraction, scanning electron microscopy, transmission electron microscopy, etc. , Seebeck coefficient, etc.
[0056] figure 2 for DHTMB 1 H NMR chart, it can be seen from the figure that the ratio of the number of hydroxyl groups to sulfhydryl groups is 1:2, which is the same as its theoretical value.
[0057] image 3 is the synchrotron radiation powder X-ray diffraction peak of Ag-DHTMB crystallite and its fitting diagram with the theoretical diffraction peak. It can be seen that the half width of the synchrotron radiation powder X-ray diffraction peak of Ag-DHTMB crystallite is very narrow, indicating that the The crystallinity of the crystal is very high. Compared with the theoretical diffrac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com