Supramolecular nano-drug for activating Hippo pathway as well as preparation method and application of supramolecular nano-drug
A nano-drug and supramolecular technology, applied in the field of nano-biomedical materials, can solve the problems of weak intracellular retention ability and weak tumor penetration ability, achieve good biocompatibility, easy clinical transformation, and improve solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
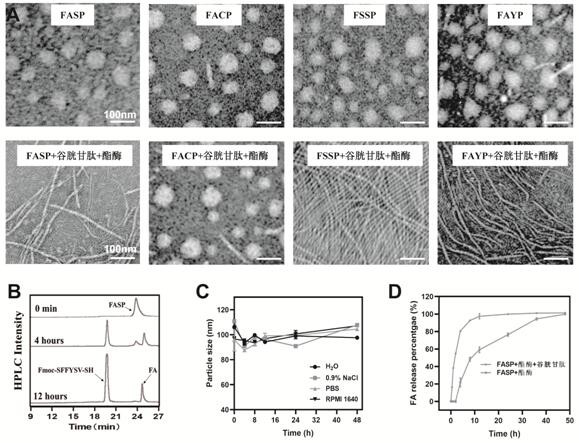
[0042] Preparation and characterization of supramolecular nanomedicines, including Fmoc-S(FA)FFYSV-SS-PEG (abbreviated as FASP), control material Fmoc-SFFYSV-SS-PEG without FA (abbreviated as FSSP), without histone deacetylation The control material Fmoc-S(FA)FFY-SS-PEG (abbreviated as FAYP) of the enzyme inhibitory peptide YSV, and the control material Fmoc-S(FA)FFYSV-CC-PEG (abbreviated as FAYP) without glutathione response FACP), taking FASP nanomedicine as an example, the specific preparation steps are as follows:
[0043] (1) Weigh 5 mg of pure polypeptide, dissolve it in 1 mL DMSO, and slowly add it dropwise to 9 mLddH in a ratio of 1:9. 2 In O / PBS, a drop of DMSO solvent containing peptides was added dropwise every 30 s, while stirring; after the dropwise addition, continued stirring at room temperature for 4 h to stabilize the micelles; the obtained product was placed in a dialysis bag (MWCO=1000 D) After dialysis for 48 h, the DMSO solvent was completely removed to o...
Embodiment 2
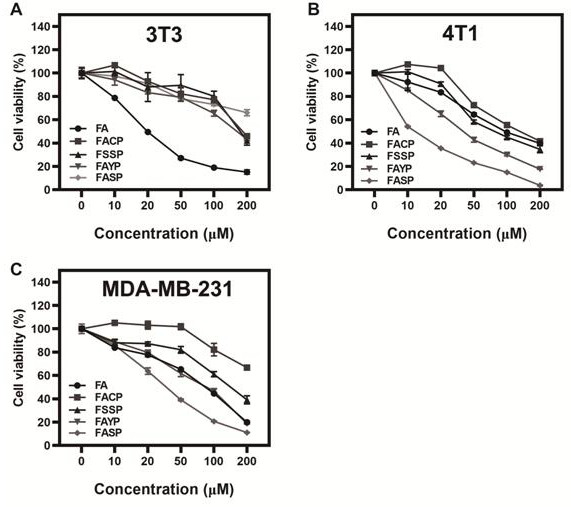
[0049] The growth inhibitory effects of the nano-drugs FASP, FSSP, FAYP, FACP and free drug FA prepared in Example 1 on tumor cells and normal cells in vitro were evaluated, and the specific implementation steps were as follows:
[0050] 1) Take MDA-MB-231, 4T1 and 3T3 cells in the logarithmic growth phase, and inoculate 6000 cells per well into a 96-well plate at 37°C, 5% CO 2 Cultured in a constant temperature incubator for 24 h;
[0051] 2) Dilute the nano-drugs FASP, FAYP, FACP, FSSP and free drug FA to a preset concentration with culture medium and incubate with the cells for 48 h;
[0052] 3) Add 10 μL of CCK-8 solution to each well under the dark condition, put it in the incubator for 2-4 hours, use a microplate reader to detect the absorbance at 450 nm, and calculate the different concentrations of each group. The survival rate of each cell after drug action;
[0053] 4) figure 2 It is shown that the nanomedicine FASP can significantly improve the growth inhibitory...
Embodiment 3
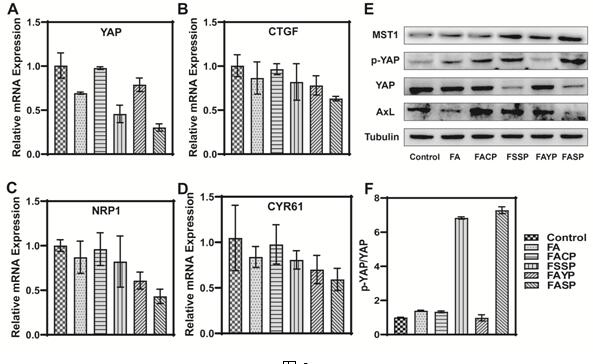
[0055] (1) Fluorescence quantitative PCR analysis was performed on the regulation of the key target genes of the Hippo pathway by the supramolecular nano-drugs and free drugs prepared in Example 1. The specific steps are as follows:
[0056]1) Take MDA-MB-231 cells in logarithmic growth phase and inoculate them in a 6 cm culture dish at a density of 100,000 per well, at 37°C, 5% CO 2 Cultured in a constant temperature incubator for 24 h;
[0057] 2) Discard the original medium and add fresh medium containing 50 μM of nano-drugs and free drugs, and incubate with cells for 48 h in the incubator;
[0058] 3) Wash the cells twice with PBS, and trypsinize to collect the cells;
[0059] 4) Wash once with PBS, centrifuge at 3000 rpm for 5 min, and discard the supernatant;
[0060] 5) Add 1 mL of 4°C pre-cooled Trizol reagent, and lyse at room temperature for 5 min;
[0061] 6) Add 200 μL of chloroform and let stand for 3 minutes;
[0062] 7) 13500 rpm, 15 min;
[0063] 8) Take t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com