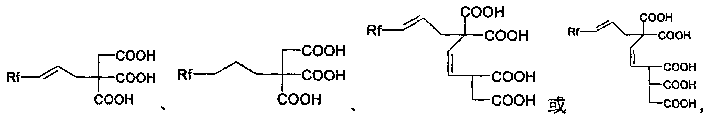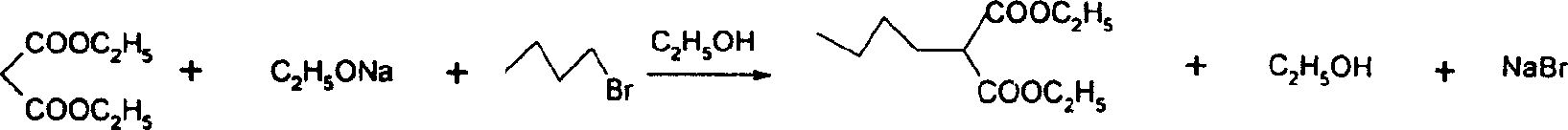Fluorine-contained polybasic carboxylic acid compound synthesis method and use thereof
A polycarboxylic acid and compound technology, which is applied in dyeing and finishing auxiliaries and fabric finishing technology, can solve the problems of limited scope of application, reduce production costs, and achieve obvious fabric anti-wrinkle, water-repellent, oil-repellent, and anti-fouling effects. The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 1. Synthesis of Compound 01
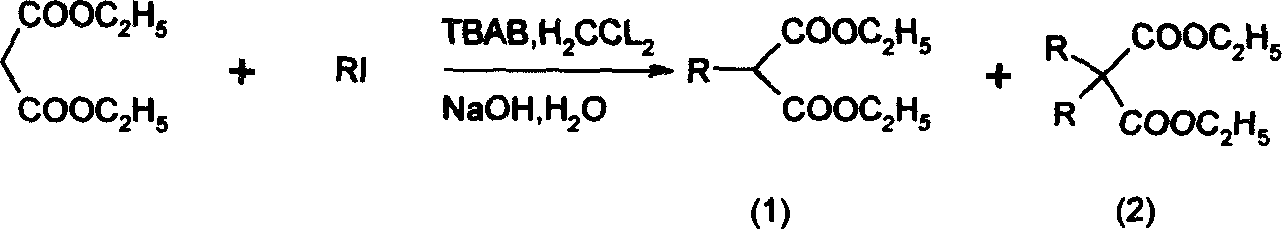
[0050] Add 15-25ml (0.1-0.3mol) of diethyl malonate, 14-17ml (0.1-0.2mol) of ethyl bromoacetate, anhydrous K 2 CO 3 20-24g (0.1-0.3mol), tetrabutylammonium chloride 0.8-1.1 (0.003-0.004mol). Electromagnetic stirring, oil bath heating to reflux state, keep 2-5 hours. After the reaction was completed, the reaction system was lowered to room temperature, distilled water was added into the three-necked flask until all the solids were dissolved, and the organic phase was separated. The aqueous phase was extracted with ethyl acetate (20ml×3), the organic phases were combined, and 10% hydrochloric acid was added dropwise until the pH of the solution was about 6. Wash the organic phase with saturated brine (20ml×2), anhydrous MgSO 4 dry. H silica gel column chromatography, chromatographic liquid ethyl acetate:petroleum ether=1:15-30, to obtain 22-26 g of the target product [A], with a yield of 80-90%. For the data of compound [A] see: Brinda...
Embodiment 2
[0059] Add PbBr successively to the three-necked flask filled with anhydrous DMF 2 (0.1468g, 0.4mmol), Al powder (0.324g, 12mmol), stirred at room temperature for 10 minutes under nitrogen protection. Join C 8 f 17 I (5.459g, 10mmol), after stirring for 4 hours at room temperature, add an appropriate amount of 10% dilute hydrochloric acid, extract with ether, dry and spin the solvent to obtain a solid. 2 o 5 07 for dehydration. Under the protection of nitrogen, the anhydrous diethyl ether solution of compound 07 (11.11 g) was slowly dropped into the newly prepared bromopropene Grignard reagent (equivalent). After dropping, use H of pH = 1.0-4.0 2 SO 4 The solution was acidified and heated to 60-90°C for hydrolysis and dehydration to obtain Compound 07. in CH 2 Cl 2 AlCl is used in the system 3 Catalytic Compound 09 and maleic anhydride (the ratio of the substance amount is 1:1.2 mol) were kept at 20-30° C. for 4 days to obtain Compound 10. Compound 10 was oxidized w...
Embodiment 3
[0067] With 1-iodoperfluorohexane (520g, 1.16mol), Na 2 S 2 o 4 (20.9g, 0.12mol), Na 2 HPO 4 (21.5g), CH 3CN (900ml) and water (300ml) were packed in a 2L autoclave, and ethylene gas was introduced to make the pressure reach 40atm. After continuing the reaction at 40-45°C for 3 hours, it was found that the ethylene pressure dropped significantly. 19 F NMR tracked the reaction and found that the raw material had completely reacted, stopped the reaction, discharged excess ethylene gas, separated the lower organic layer, washed with water, washed with brine, and anhydrous Na 2 SO 4 Drying and distillation gave compound 12, 1-iodo-1H,1H,2H,2H-perfluoroalkyloctane (525g, yield 95%, boiling point 90-92°C / 45mmHg). Compound 13 was obtained by reacting compound 12 with excess KOH in ethanol system. in CH 2 Cl 2 AlCl is used in the system 3 Compound 13 was catalyzed with maleic anhydride and aconitic acid (the ratio of the substances was 1:1.2 mol), and kept at 20-30° C. for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com