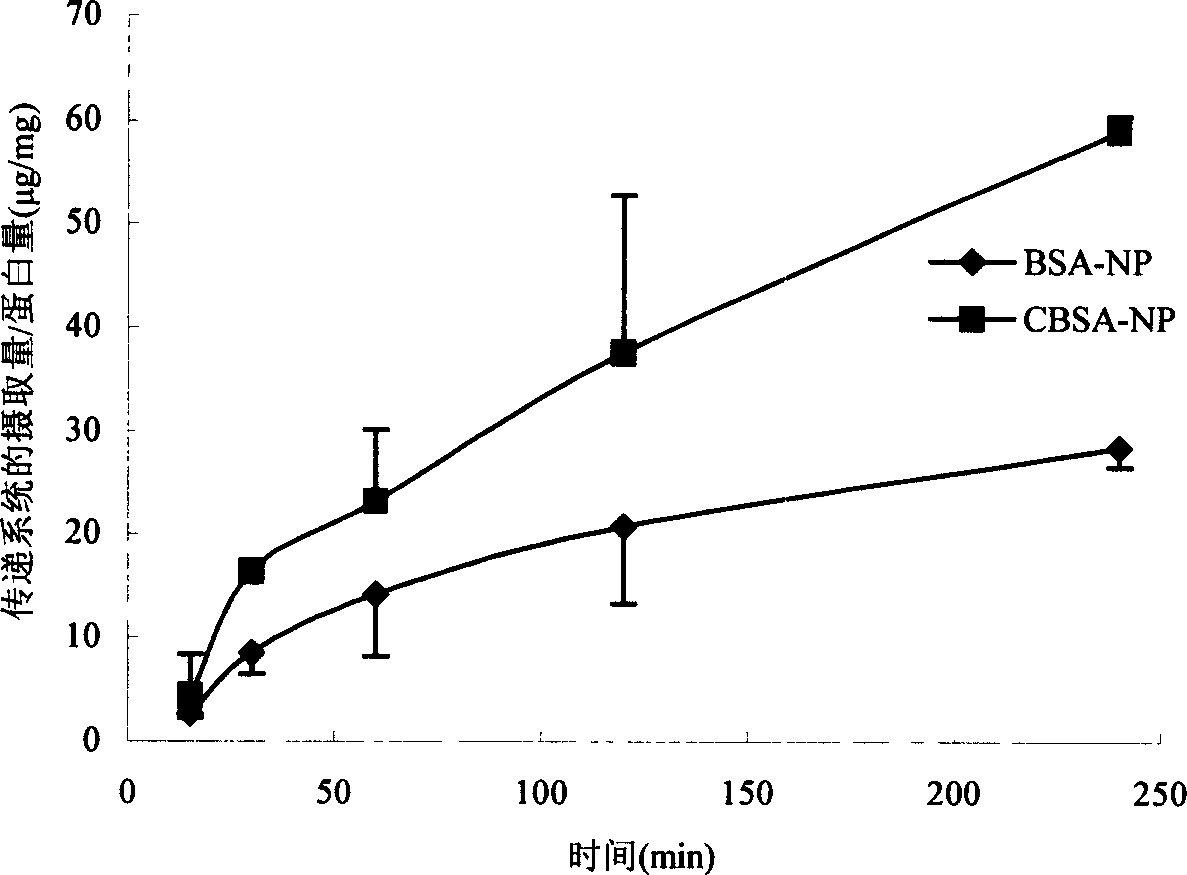
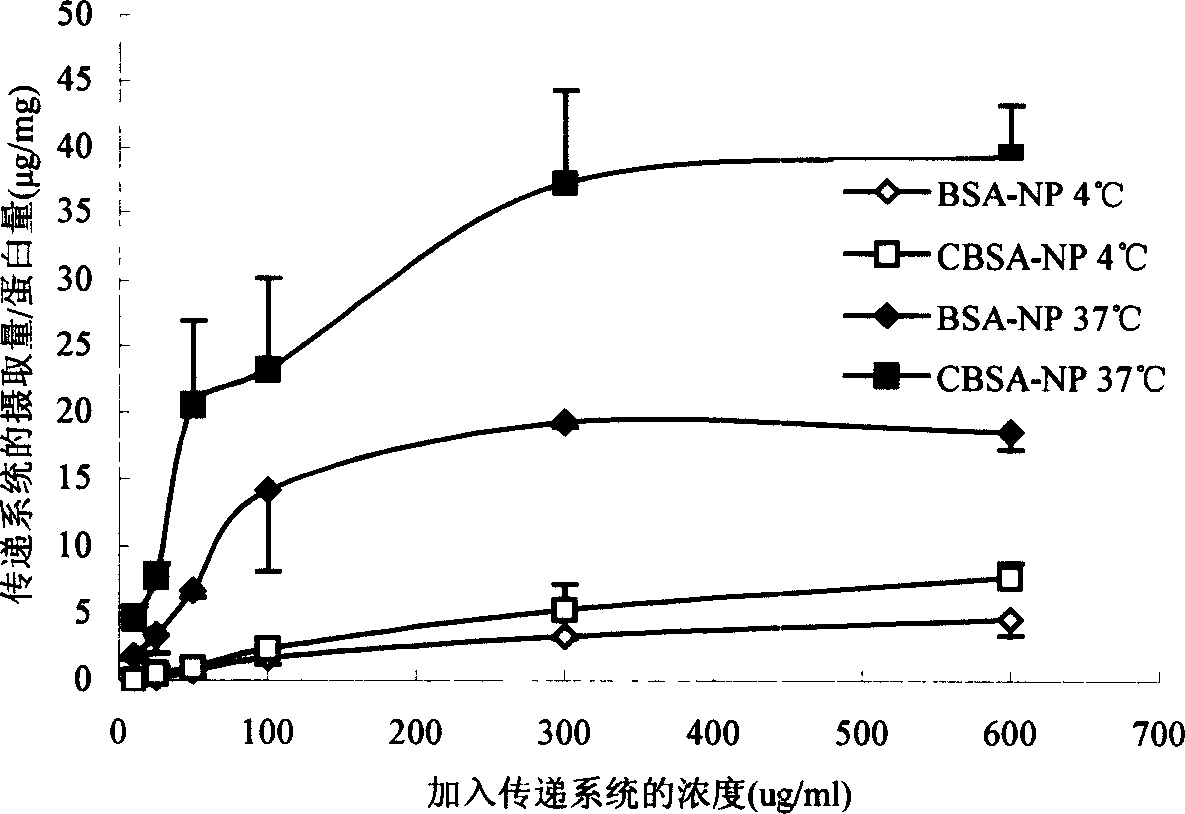
Medicinal transferring system for intracephalic transference of medicine
A technology for delivery systems and drugs, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and non-active ingredients in polymer compounds, etc. The effect of reducing the dosage, overcoming the instability of modification, and convenient preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of a drug delivery system loaded with fluorescent dye 6-coumarin
[0032] 1) Slowly add 250ml of 0.9mol / L ethylenediamine to 4ml of 50% bovine serum albumin (BSA), stir while adding, adjust the pH to 4.75 with hydrochloric acid, add 360mg of EDAC, stir at room temperature for 2 hours, then add 1.3ml 4mol / L acetate buffer solution (pH 4.75), the solution was concentrated to 25ml, dialyzed for 72h, and freeze-dried to obtain CBSA. The molecular weight of the synthesized CBSA was identified by SDS-PAGE, and the isoelectric point was identified by isoelectric focusing electrophoresis (IEF). IEF results showed that the isoelectric point of CBSA was between 8-9, and SDS-PAGE showed that the molecular weight of CBSA was close to that of BSA, and the molecular weight was about 66000D.
[0033] Take 10 μl of 10 mg / ml S-acetyl-thioglycolic acid-N-succinimide (SATA) in dimethyl sulfoxide solution, add CBSA 1 mg (1 mg / ml) in HEPES solution (pH 7.4), stir for...
Embodiment 2
[0054] Example 2: Preparation of drug delivery system loaded with fluorescent dye 6-coumarin
[0055] 1) The method for preparing CBSA and mercaptolated CBSA is the same as in Example 1
[0056] Preparation of PEGylated nanoparticles loaded with 6-coumarin
[0057] Add 50 μl distilled water to 1 ml containing 24 mg monomethoxypolyethylene glycol 3000 - Polylactic acid 28600 (MPEG-PLA), 2.4mg maleimide-polyethylene glycol 3400 - Polylactic acid 23900 (maleimide-PEG-PLA) block copolymer and 15μg 6-coumarin in dichloromethane, 60W continuous ultrasonic 30s to prepare w / o type colostrum. Add colostrum into 2ml of 1% sodium cholate solution, and perform ultrasonication at 60W 30 times (1s / time) intermittently to obtain w / o / w type double emulsion. Add the double emulsion to 38ml of 0.5% sodium cholate solution, stir for 1min, remove methylene chloride by rotary evaporation at 40°C, centrifuge at 15000rpm at 4°C for 45min, remove the supernatant, and resuspend with 0.01mol / L pH7...
Embodiment 3
[0060] Example 3: Preparation of a drug delivery system loaded with the cerebral vasodilator nimodipine
[0061] 1) Slowly add 250ml of 0.9mol / L hexamethylenediamine to 4ml of 50% human serum albumin (HSA), stir while adding, adjust the pH to 4.75 with hydrochloric acid, and add 360mg of EDAC. After stirring at room temperature for 2 h, 1.3 ml of 4 mol / L acetate buffer (pH 4.75) was added. The solution was concentrated to 25ml, dialyzed for 72h, and freeze-dried to obtain CHSA. IEF showed that the isoelectric point of CHSA was between 8-9, and SDS-PAGE showed that the molecular weight of CHSA was close to that of HSA.
[0062] Take 150 μl of 1mg / ml Traut's reagent (2-iminothiolane) in borate buffer (pH 8.0), add CHSA 2mg (2mg / ml) in borate buffer (pH 8.0), stir for 60min, and pass through the reaction solution The buffer salt of Sephadex G-100 gel column was replaced with PBS at pH 7.4 to obtain thiolated CHSA. The degree of sulfhydrylation of CHSA determined by Ellman's re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Number average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com