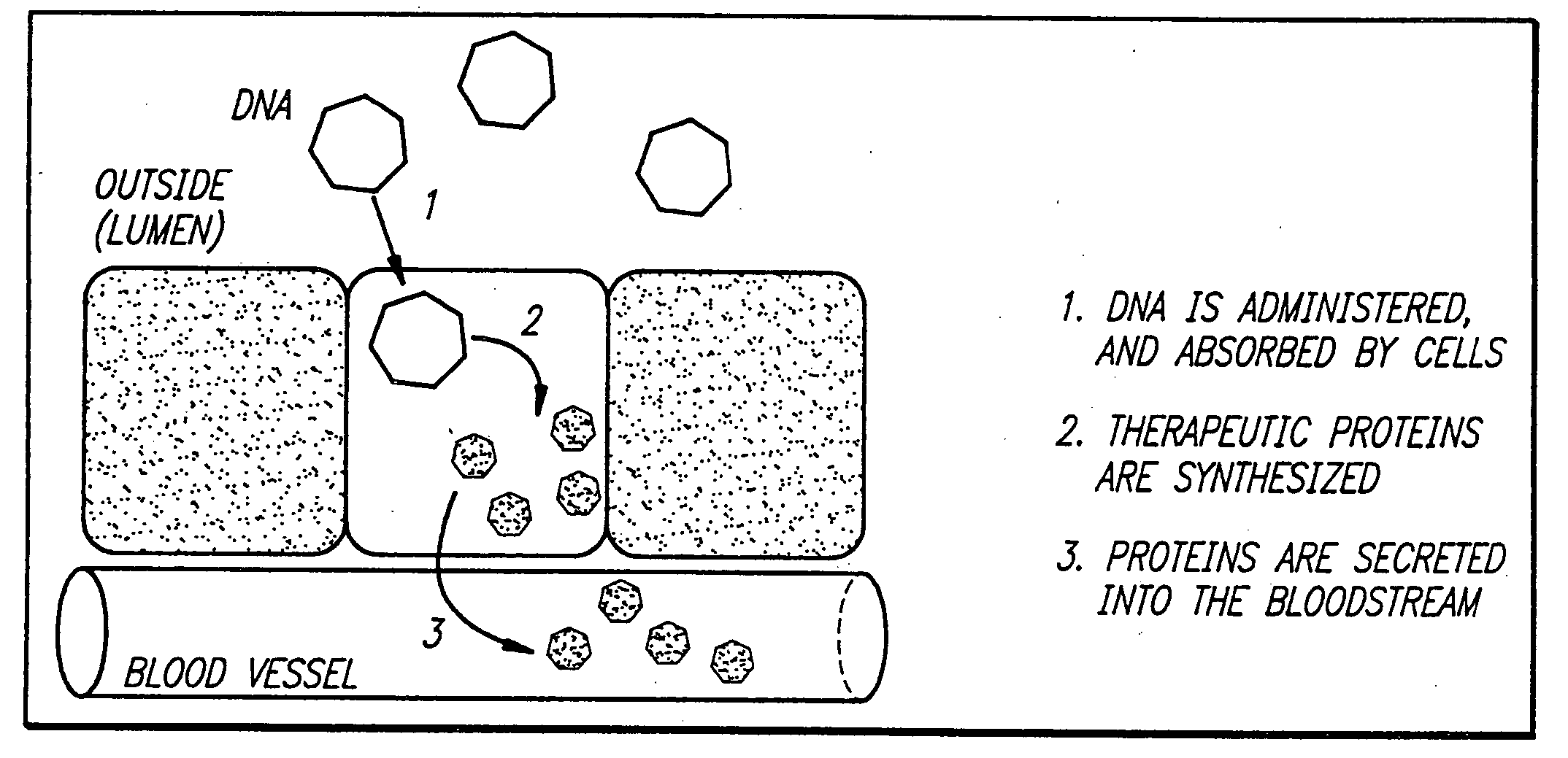
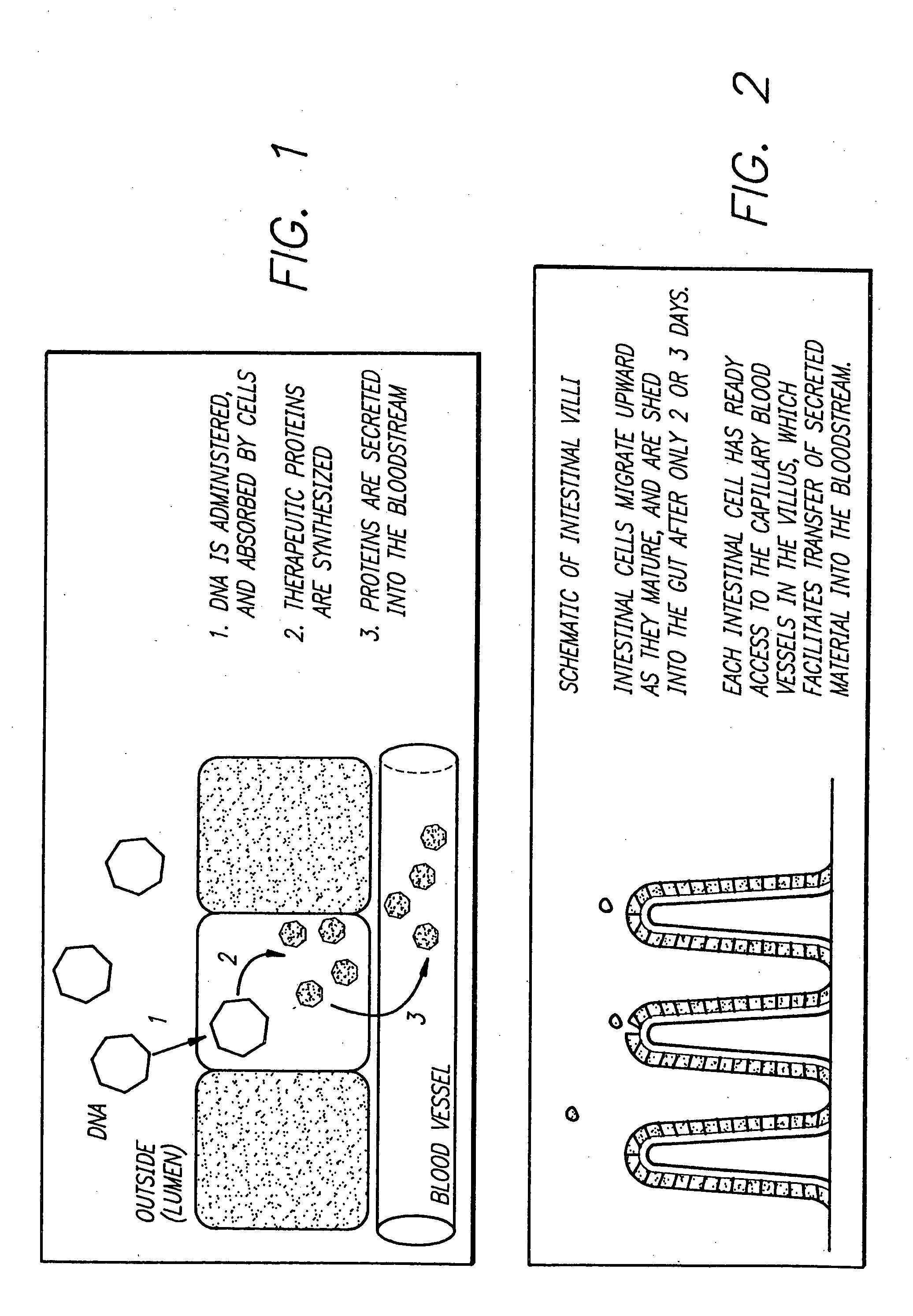
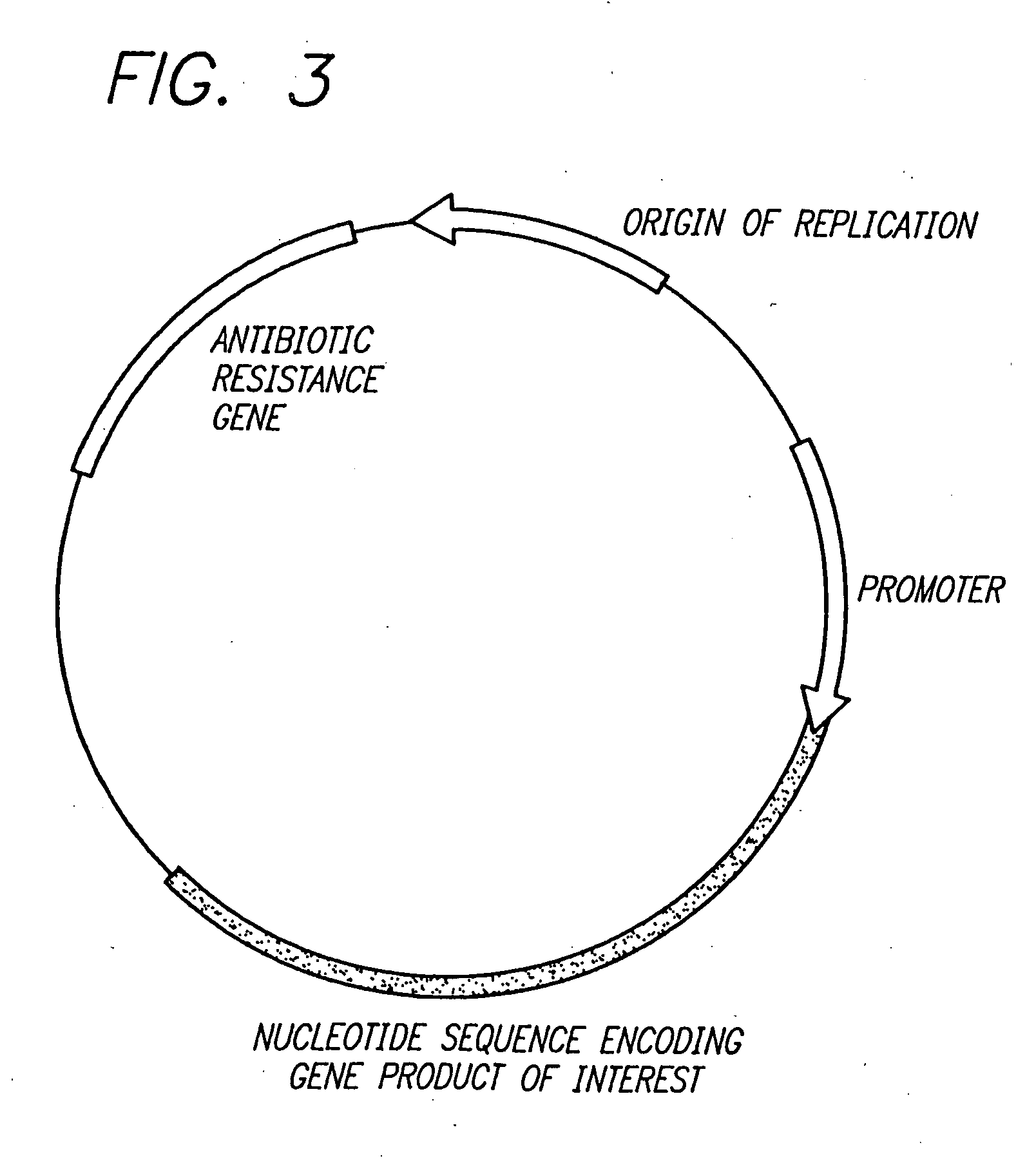
Delivery of therapeutic gene products by intestinal cell expression
a technology of intestine cells and gene products, applied in the field of drug delivery, can solve the problems of limiting the commercialization of these drugs, difficult and expensive production of proteins in bioactive form, and limited use of these drugs, so as to achieve rapid turnover, advantageous dose control, and easy modification or modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0112] Construction of Vectors Expressing Human Growth Hormone (hGH) for Intestinal Cell Transformation
[0113] Four constructs for expression of human growth hormone (hGH) were prepared using techniques well known in the art (see, for example, Sambrook et al., supra). The first construct, pFGH, contains the genomic hGH DNA sequence inserted in the commercially available vector pBLUESCSRIPT SK+™ (Stratagene, La Jolla Calif.) (FIG. 2). Because the hGH coding sequence is not linked to a promoter, this vector provides for no or only low-level hGH expression. Thus, the pFGH construct serves as a negative control for hGH expression in the intestine. The second construct, pFGH.CMV, was constructed by operably inserting the promoter from the immediate early gene of human CMV upstream of the genomic hGH sequence of the pFGH vector (FIG. 3). The third construct, pFGH.chymo, was constructed by operably inserting the rat chymotrypsin B gene promoter upstream of the genomic hGH sequence of the p...
example 2
[0114] In Vivo Gene Transfer of DNA Encoding Human Growth Hormone by Introduction of Naked DNA into the Intestinal Lumen
[0115] pFGH.CMV was used to transform intestinal epithelium of approximately 300 g adult rats (pFGH.CMV 10 rats; pFGH.CMV with lipofectin, 4 rats; pFGH.CMV with polycationic dendrimers, 4 rats; negative control (PBS), 1 rat; and negative control (no surgery), 8 rats.
[0116] The rats were anesthetized with pentobarbital. A laparotomy was performed and the upper duodenum or terminal ileum identified. A 5 cm length of intestine was ligated, a small aliquot of venous blood was obtained, and 400 μl of phosphate-buffered saline (PBS) containing pFGH.CMV, or 400 μl of PBS alone (negative control no. 1), were slowly injected or infused into the intestine and left in place for 15 min. The amount of solution used produces a slight expansion of the bowel. The vector-containing solutions were composed of 20-200 μg DNA per 400 μl in PBS; 32 μg DNA per 400 μl in PBS with 6% lip...
example 3
[0119] Construction of Vectors Expressing Human Insulin (hIns) for Intestinal Cell Transformation
[0120] Two constructs for expression of human insulin and a human insulin mutant were prepared using techniques well known in the art (see, e.g., Sambrook et al., supra). The first construct, pBAT14.hins, contains a cDNA sequence encoding human insulin which is inserted in the commercially available vector pBLUESCRIPT SK+™ (Stratagene, La Jolla Calif.) (FIG. 6A). The human insulin encoding sequence is operably linked to a promoter from the immediate early gene of human CMV, which is positioned upstream of the first intron of human β-globin and of the human insulin-encoding cDNA sequence. The second construct, pBAT16.hInsG1.M2, was constructed by operably linking the CMV promoter upstream of a nucleotide sequence encoding a mutant of human insulin (FIG. 6B). The mutation in the human insulin mutant changes the second protease site between peptides C and A into a furin recognition site in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
| flexibility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com