Methods for sterilizing preparations of glycosidases
a glycosidases and preparation technology, applied in the field of glycosidases preparation sterilization, can solve the problems of ineffective glycosidases, poor absorption of ingested meat by people without peptic activity in the stomach, and useless substances as nutrients, etc., to reduce the temperature of preparations, and reduce the residual solvent content of preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
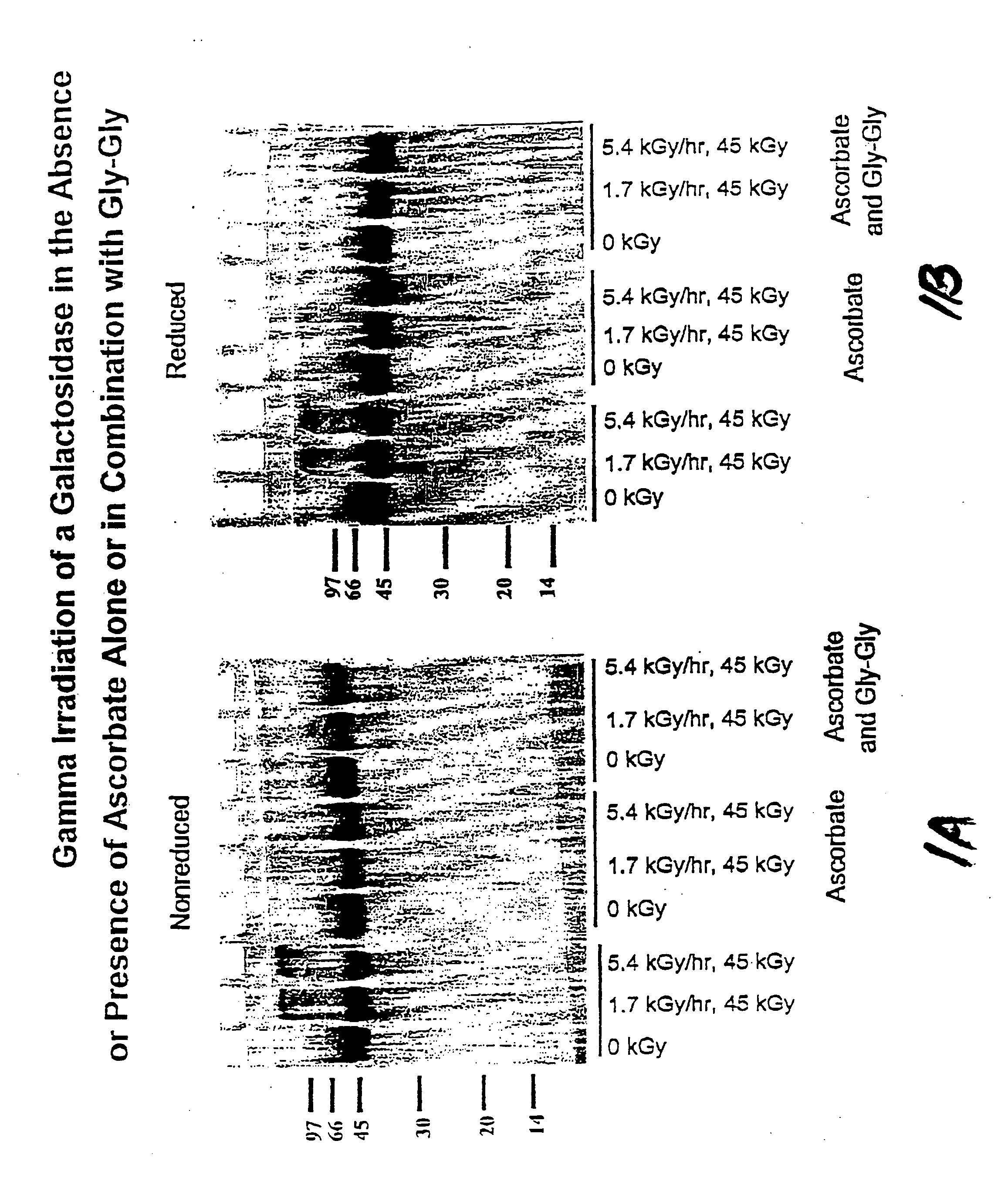
[0109] In this experiment, the protective effect of ascorbate (200 mM) and a combination of ascorbate (200 mM) and Gly-Gly (200 mM) on a frozen galactosidase preparation was evaluated.
[0110] Method
[0111] In glass vials, 300 μl total volume containing 300 μg of enzyme (1 mg / ml) were prepared with either no stabilizer or the stabilizer of interest. Samples were irradiated with gamma radiation (45 kGy total dose, dose rate and temperature of 1.616 kGy / hr and −21.5° C. or 5.35 kGy / hr and —21.9° C.) and then assayed for structural integrity.
[0112] Structural integrity was determined by SDS-PAGE. Three 12.5% gels were prepared according to the following recipe: 4.2 ml acrylamide; 2.5 ml 4×-Tris (pH 8.8); 3.3 ml water; 100 μl 10% APS solution; and 10 μl TEMED (tetramethylethylenediamine). This solution was then placed in an electrophoresis unit with 1×Running Buffer (15.1 g Tris base; 72.0 g glycine; 5.0 g SDS in 1 l water, diluted 5-fold). Irradiated and control samples (1 mg / ml) were ...
example 2
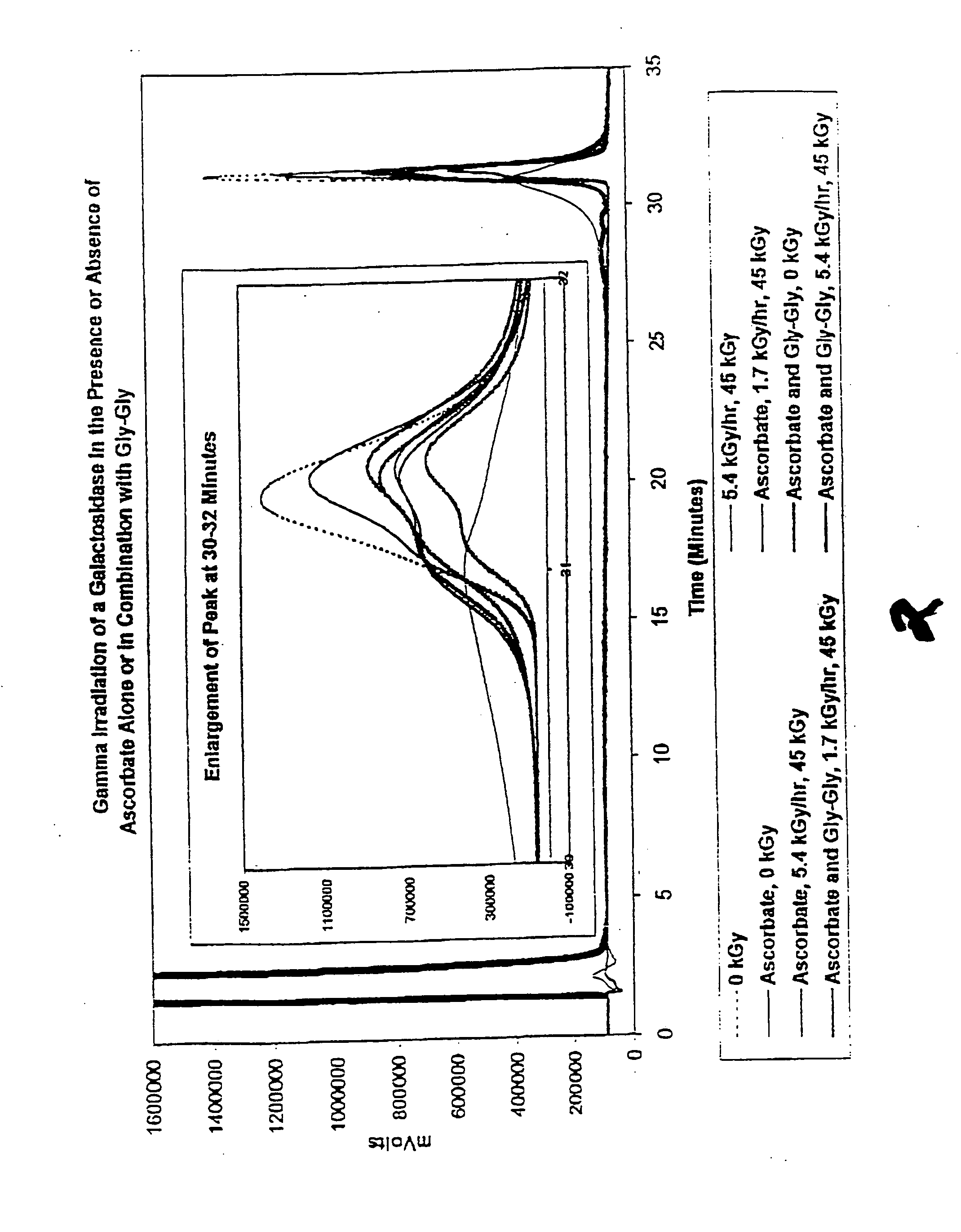
[0115] In this experiment, the protective effect of ascorbate (200 mM) and a combination of ascorbate (200 mM) and Gly-Gly (200 mM) on a frozen galactosidase preparation was evaluated.
[0116] Method
[0117] Samples were prepared in 2 ml glass vials, each containing 52.6 μl of a glycosidase solution (5.7 mg / ml), and either no stabilizer or a stabilizer of interest, and sufficient water to make a total sample volume of 300 μl. Samples were irradiated with gamma radiation (45 kGy total dose, dose rate and temperature of either 1.616 kGy / hr and −21.5° C. or 5.35 kGy / hr and —21.9° C.) and then assayed for structural integrity.
[0118] Structural integrity was determined by reverse phase chromatography. 10 μl of sample were diluted with 90 μl solvent A and then injected onto an Aquapore RP-300 (c-8) column (2.1×30 mm) mounted in an Applied Biosystems 130A Separation System Microbore HPLC. Solvent A: 0.1% trifluoroacetic acid; solvent B: 70% acetonitrile, 30% water, 0.085% trifluoroacetic ac...
example 3
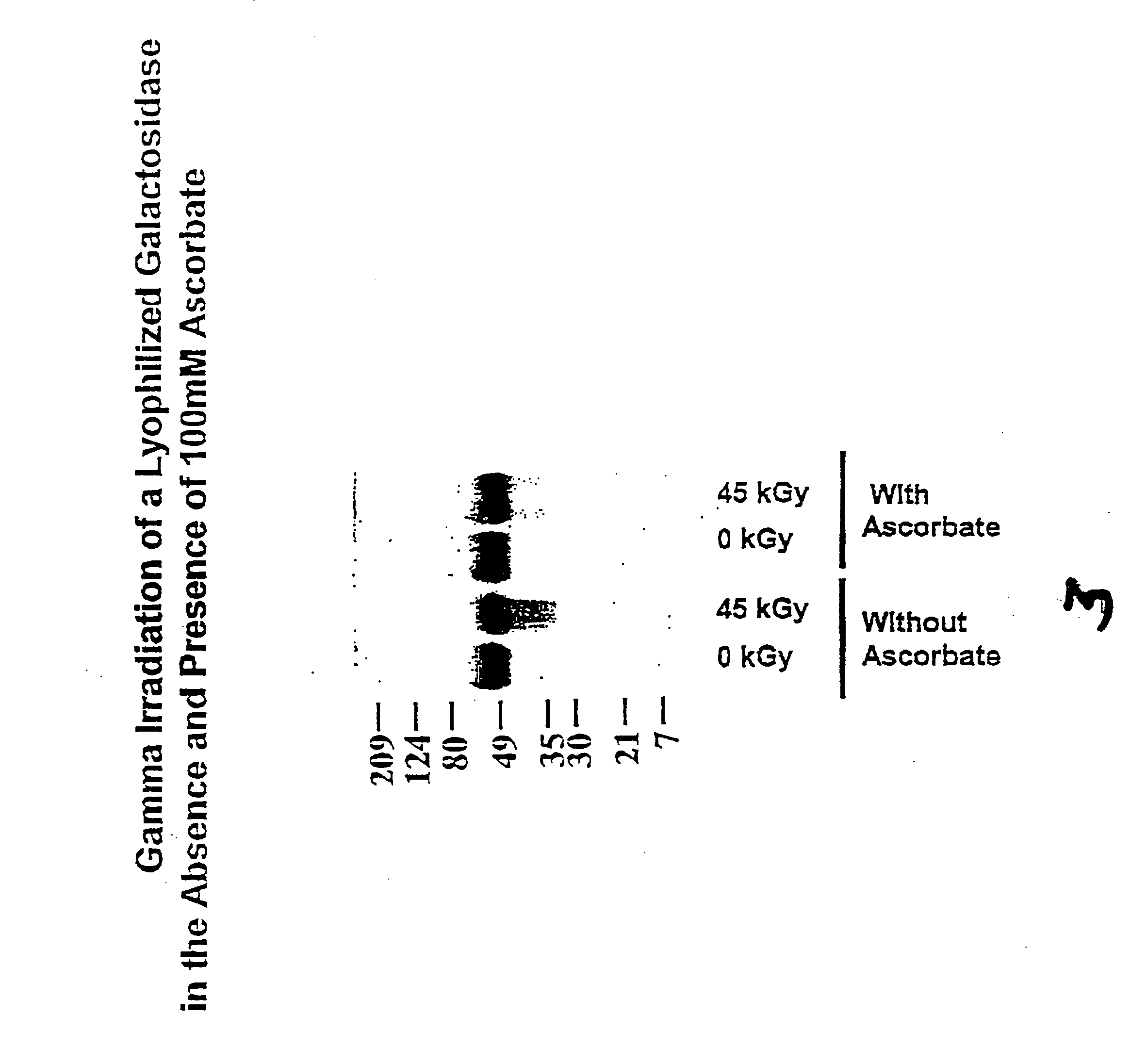
[0121] In this experiment, lyophilized galactosidase preparations were irradiated in the absence or presence of a stabilizer (100 mM sodium ascorbate).
[0122] Method
[0123] Glass vials containing 1 mg of enzyme were prepared with either no stabilizer or 100 mM sodium ascorbate (50 μl of 2M solution) and sufficient water to make 1 ml of sample. Samples were lyophilized, resulting in the following moisture levels: galactosidase with stabilizer, 3.4%; galactosidase without stabilizer, 3.2%. Lyophilized samples were irradiated with gamma radiation (45 kGy total dose at 1.8 kGy / hr and 4° C.) and then assayed for structural integrity.
[0124] Structural integrity was determined by SDS-PAGE. In an electrophoresis unit, 6 μg / lane of each sample was run at 120V on a 7.5%-15% acrylamide gradient gel with a 4.5% acrylamide stacker under non-reducing conditions.
[0125] Results
[0126] Lyophilized galactosidase samples irradiated to 45 kGy in the absence of a stabilizer showed significant recovery...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com