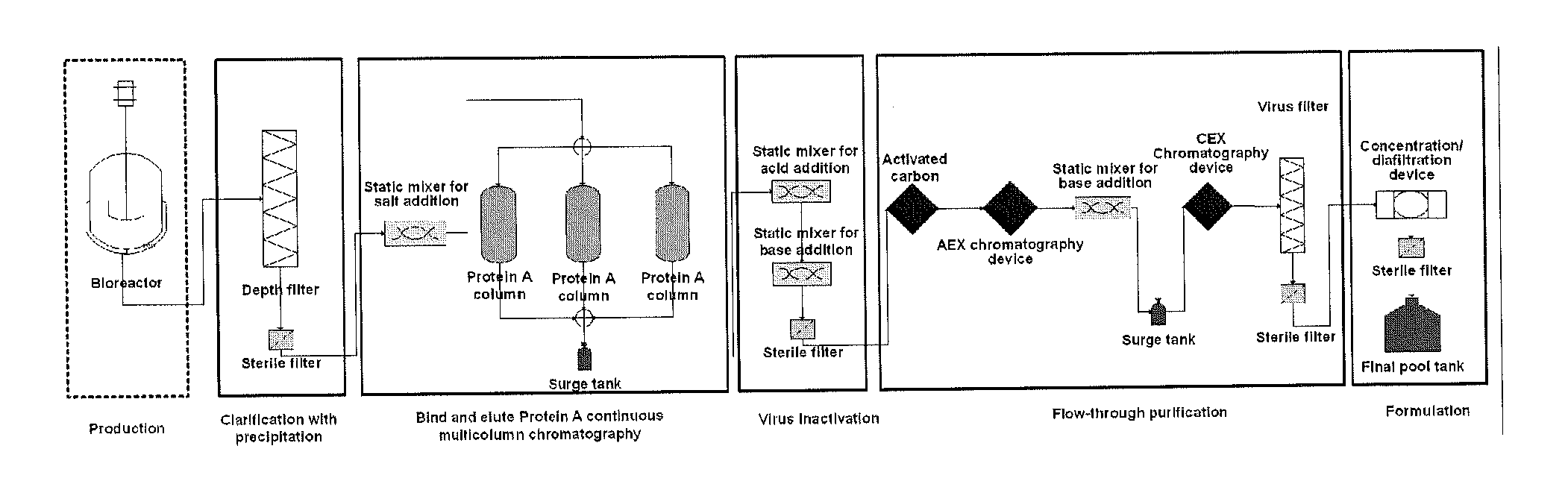
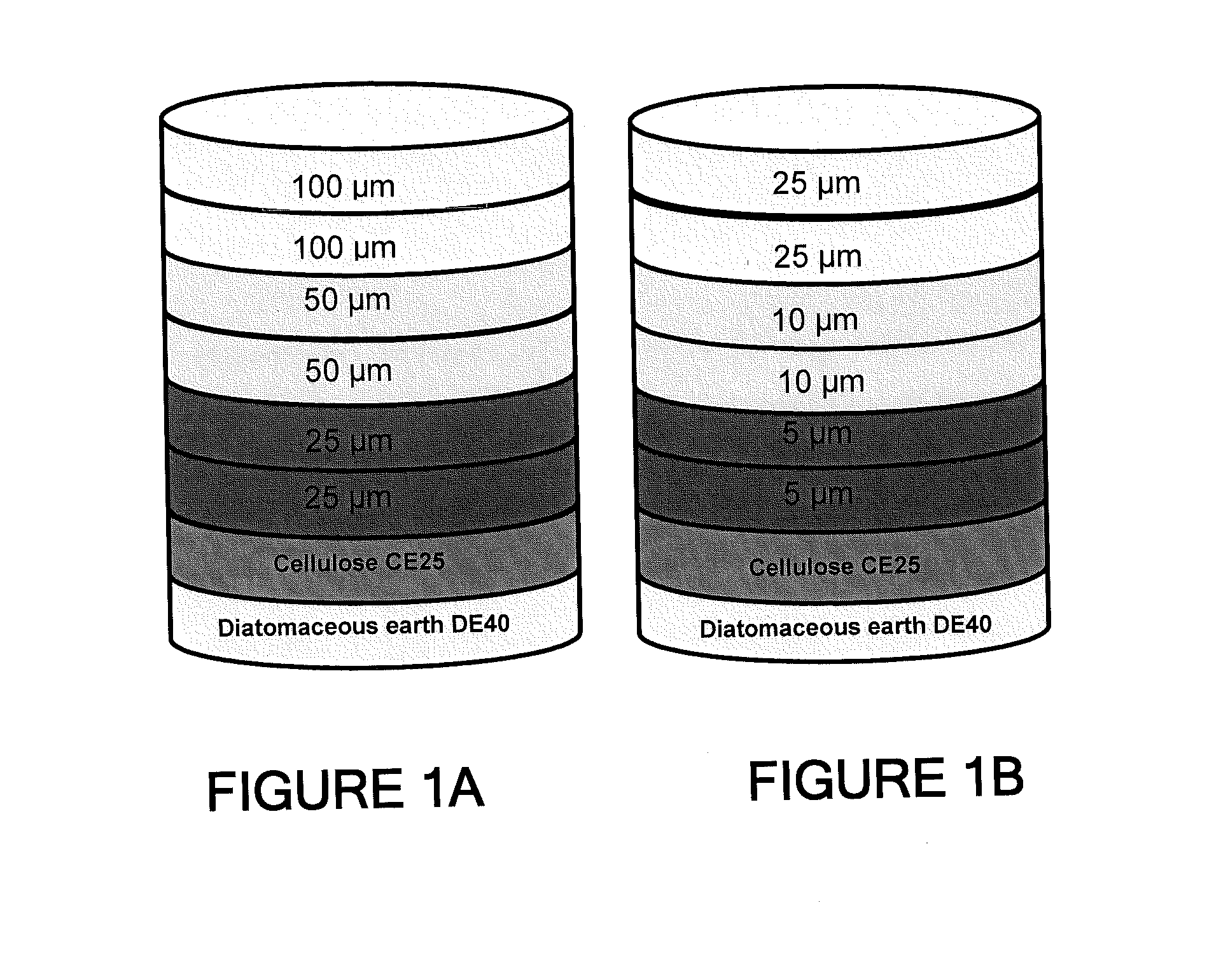
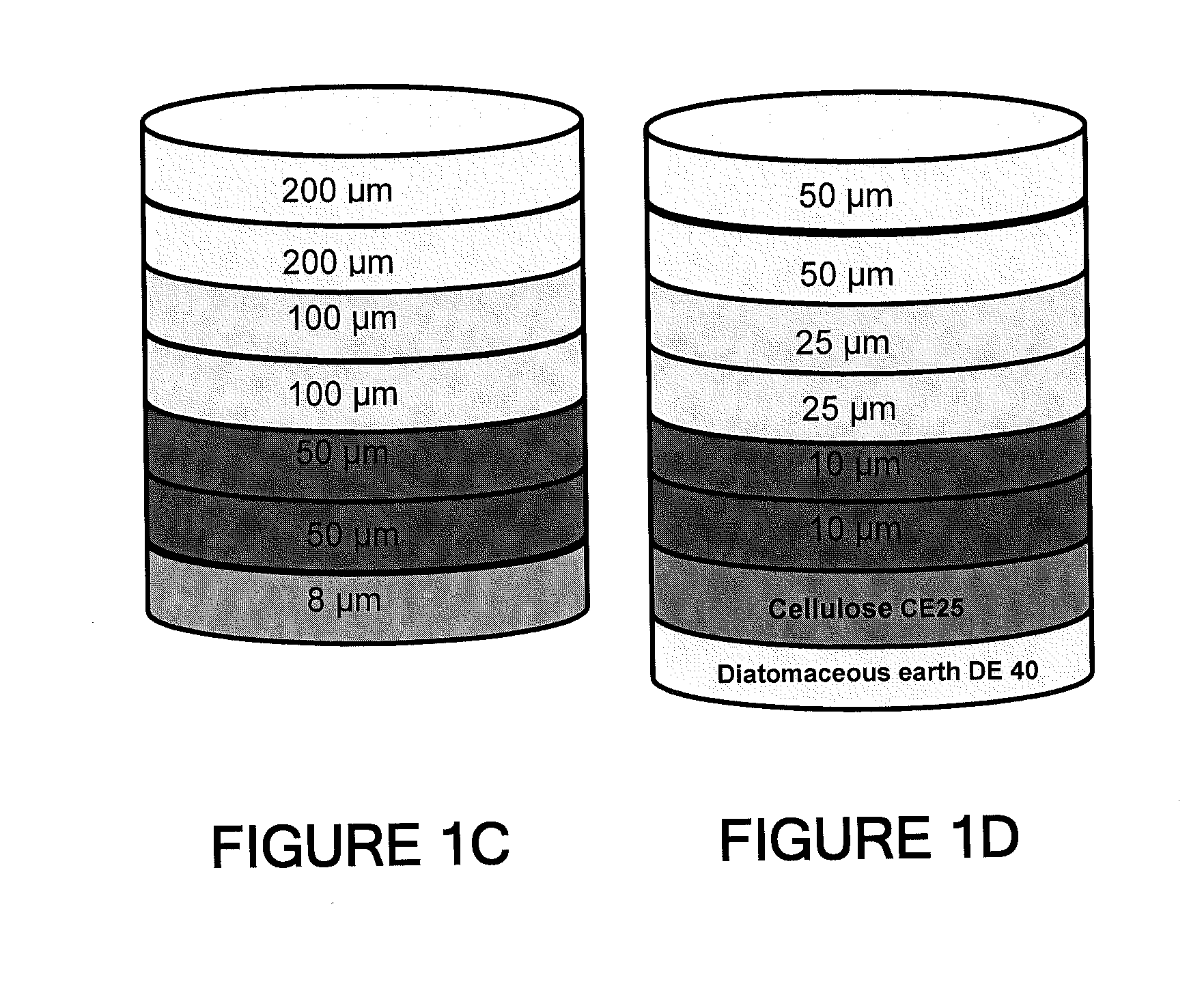
Depth Filters For Disposable Biotechnological Processes
a biotechnological process and filter technology, applied in chemical/physical processes, peptides, water treatment, etc., can solve the problems of increased impurity load, difficulty in sterile filtration, and large amount of product,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Unclarified Non-Expressing Cell Culture Fluid (CCF)
[0172]In a representative experiment, cells derived from an expressing Chinese Hamster Ovary (CHO) cell line were grown in a 10 L bioreactor (New Brunswick Scientific, Edison, N.J.) to a density of 10×106 cells / mL and harvested at 80% viability. Monoclonal antibody (mAb) titer was determined to be 0.8 g / L. The level of host cell proteins (HCP) was found to be 200,000 ng / mL using an ELISA assay (Cygnus Technologies, Southport, N.C., #3G ELISA). The pH of the unclarified cell culture was pH 6.9.
example 2
Preparation of Multivalent Ion Stimulus Sensitive Polymer
[0173]10 g of polyallylamine (PAA) (Nittobo Medical Co., Ltd., Tokyo, Japan 150 kD; 40% wt / wt) is placed in a 100 mL round bottom flask and a solution of 3.34 g of sodium hydroxide (1.2 Eq. per monomer) in 25 mL H2O is added at room temperature under magnetic stirring and in small amounts. Benzyl chloride (2.30 g, 2.09 mL) is then added, stirred for few minutes at room temperature and then heated to 60° C. overnight for 17 hours. The reaction is then cooled to room temperature and solvent is removed resulting in polymer precipitation. The precipitated polymer is washed with water and stirred in 1M aqueous AcOH solution (40 ml) until complete solubilization is achieved. The solution is then diluted with H2O to a final volume of 400 ml (1% polymer solution), potassium dibasic phosphate (K2HPO4) (3.48 g) is added under stirring and pH of the solution is adjusted to pH 6.8 to precipitate the modified polymer. The polymer is collec...
example 3
Smart Polymer (SmP) Treatment of CHO-S Feed
[0174]In order to flocculate the cell culture with SmP, a 500 ml sample of cell culture broth from Example 1 was added to a 1000 ml media bottle. While stirring, a sample of polymer concentrate from, Example 2 to the desired polymer dose (wt %), typically 0.2%. The solution was allowed to mix for 15 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| total thickness | aaaaa | aaaaa |
| mean particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com