Plasma modified medical devices and methods
a technology of medical devices and plasma, applied in chemical vapor deposition coatings, magnetic recording, record information storage, etc., can solve the problems of affecting the safety of patients, so as to increase the apoptosis of smooth muscle cells, and increase the apoptosis of coronary artery endothelial cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Stents
[0095]Stainless steel coronary artery stents when unexpanded had dimensions of 1.6 mm (diameter)×12 mm (length) with a total exposed wire surface area of 20.66 mm2. The stents were cleaned with a 2% (v / v) Detergent 8 solution for 30 min at 50° C. in an ultrasonic bath. The stents were then sonicated in distilled water for 30 min at 50° C. Stents were given a final rinse with distilled water and dried in an oven at 50° C. for 30 min.
[0096]The stents were then threaded through an electrically conductive metal wire that had been attached to aluminum panels with a surface area 15.3 cm×7.6 cm, using silver epoxy. For DC treatment groups, we used an oxygen pretreatment step (1 sccm oxygen, 50 mTorr, 20 W DC, 2 min) followed by TMS plasma polymer deposition (1 sccm TMS, 50 mTorr, 5 W DC, 15 s) and a 2:1 ammonia / oxygen plasma surface modification treatment for 2 min at 50 mTorr and 5 W DC. For RF treatment groups, we used an oxygen pretreatment step (1 sccm oxygen, 50 m...
example 2
Water Contact Angle of Plasma Coated Stainless Steel Wafers
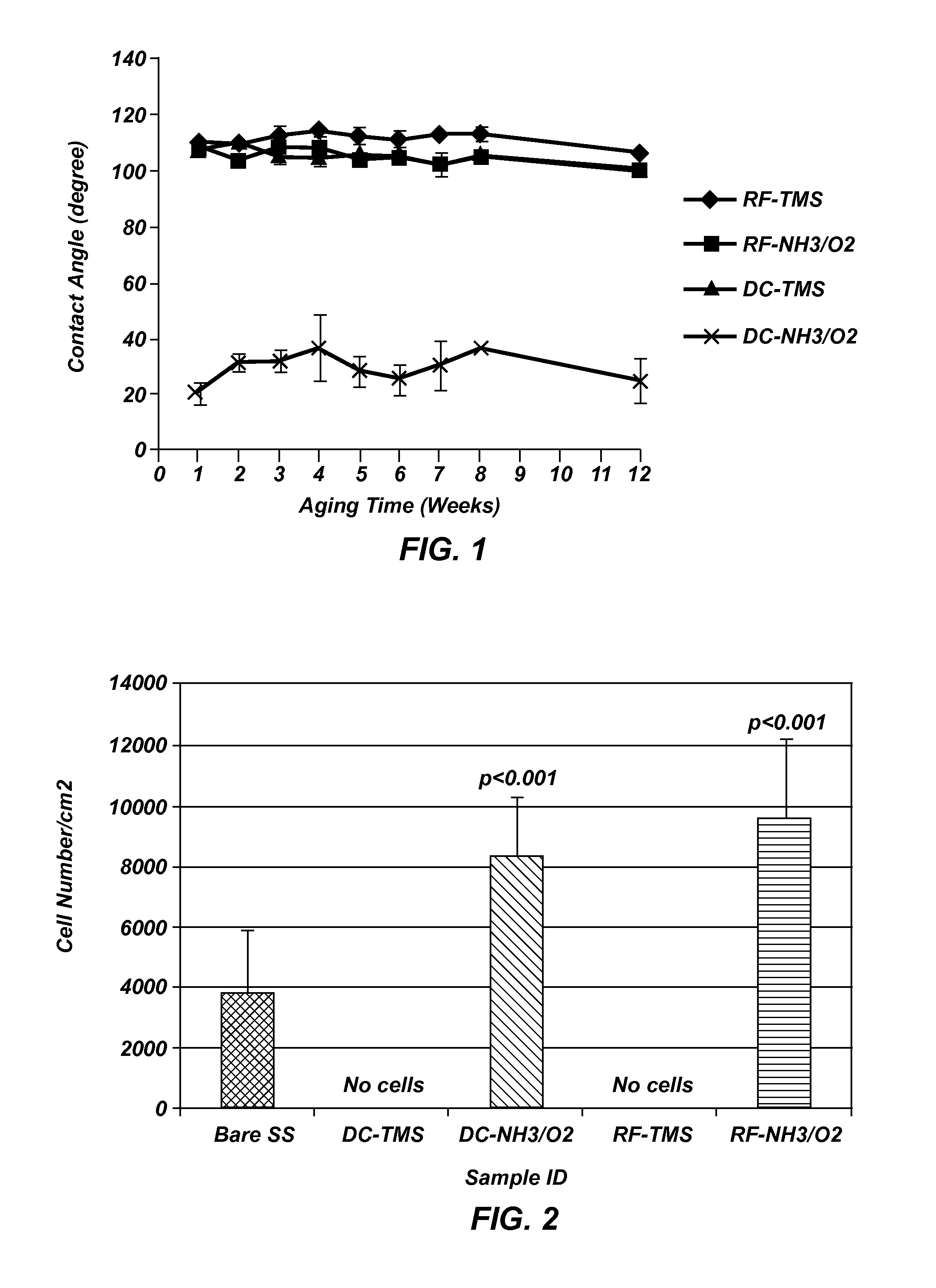
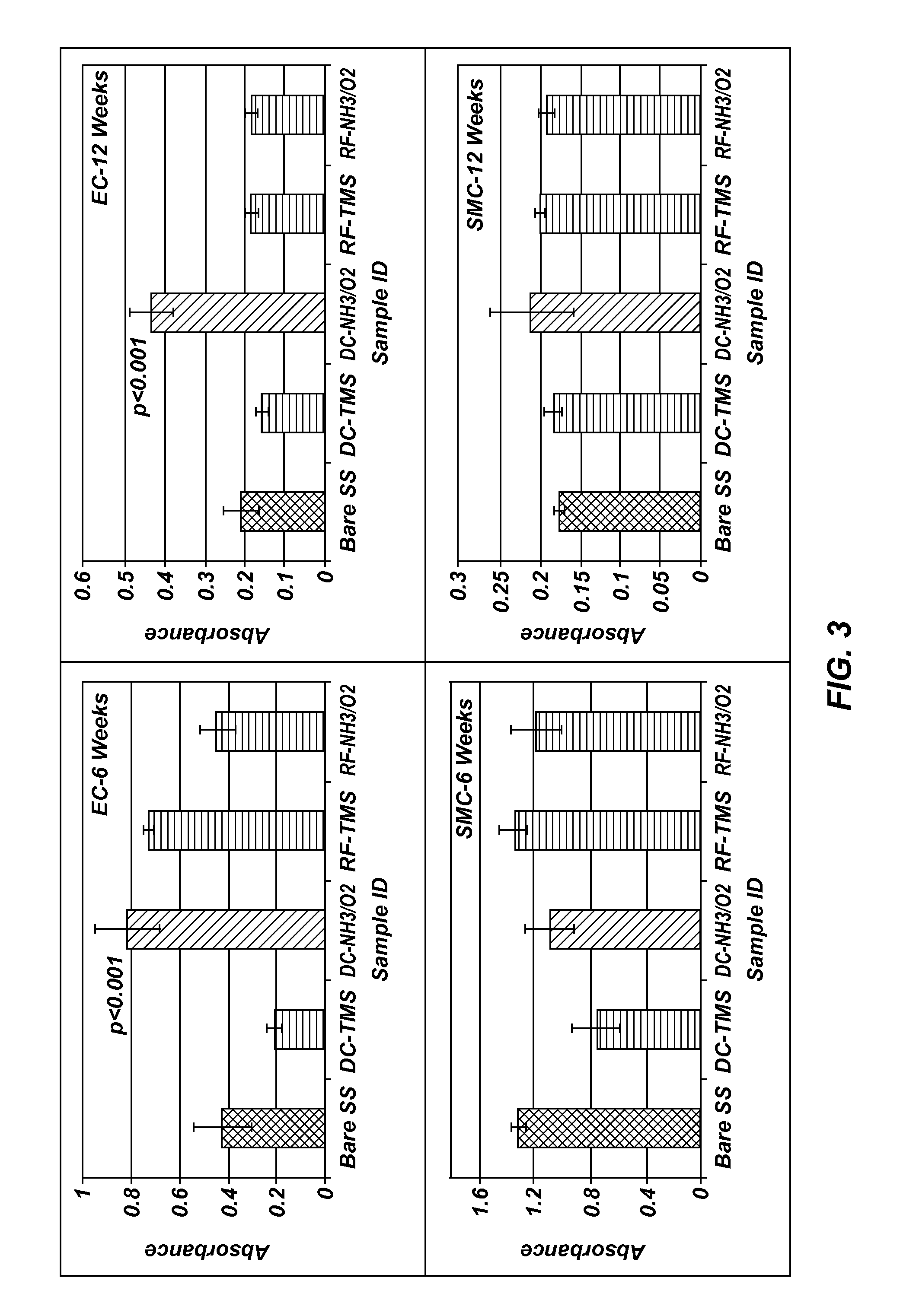
[0097]Measurements were taken on plasma coated wafers for up to 12 weeks following the plasma coating to evaluate the long teen stability. The results indicated the plasma coated surfaces tend to stabilize at about two weeks after plasma processing, and the wafers coated with TMS followed by NH3 / O2 plasma treatment using DC plasma (FIG. 1) remained very hydrophilic at 12 weeks after the plasma coating process as compared to uncoated controls, indicating long-lasting surface bioactivity generated by the plasma coating process.
example 3
Plasma Coating Adhesion to Substrate Surface and Coating Integrity
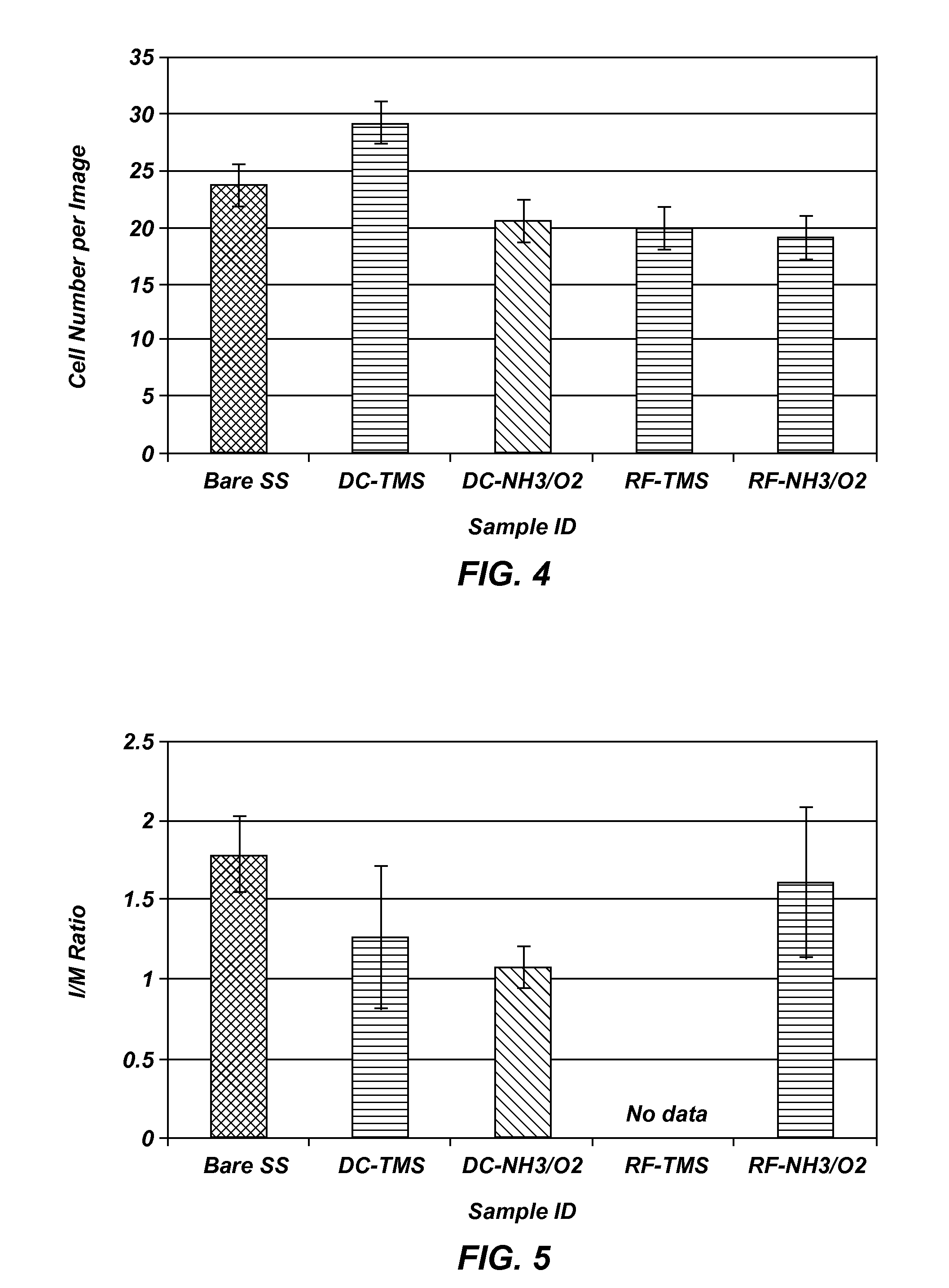
[0098]A cross-hatch was made using a razor blade on plasma coated stainless steel wafers followed by a Scotch® tape pull test. Visual inspection showed that there was no coating coming off the cross-hatched or its surrounding area, indicative of strong adhesion to the underlying surface, which warrants the coating integrity when flexed during stent crimping, navigation and expansion in clinical application.
[0099]Stainless steel stents of generic design in the dimension of Ø1.6 mm×12 mm (diameter×length) before dilation were used for the coating cracking test. After plasma coatings, the stents were imaged using an optical microscope at 20× and 50× magnifications. Following imaging, the samples were expanded with a balloon catheter (monorail™ Maverick PTCA Dilatation Catheter, Boston Scientific, Natick Mass.) and inflated to 3.0 mm in diameter; the stents were then visualized again via optical microscopy and Scanning El...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| contact angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com