Electrochemical conversion of anhydrous hydrogen halide to halogen gas using a membrane-electrode assembly or gas diffusion electrodes
a technology of anhydrous hydrogen halide and electrodes, which is applied in the field of electrochemical cells and a process for converting anhydrous hydrogen halide to halogen gas, can solve the problems of increasing the power cost per unit of cl.sub.2, and achieve the effects of reducing operating costs, increasing current density, and reducing capital investmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
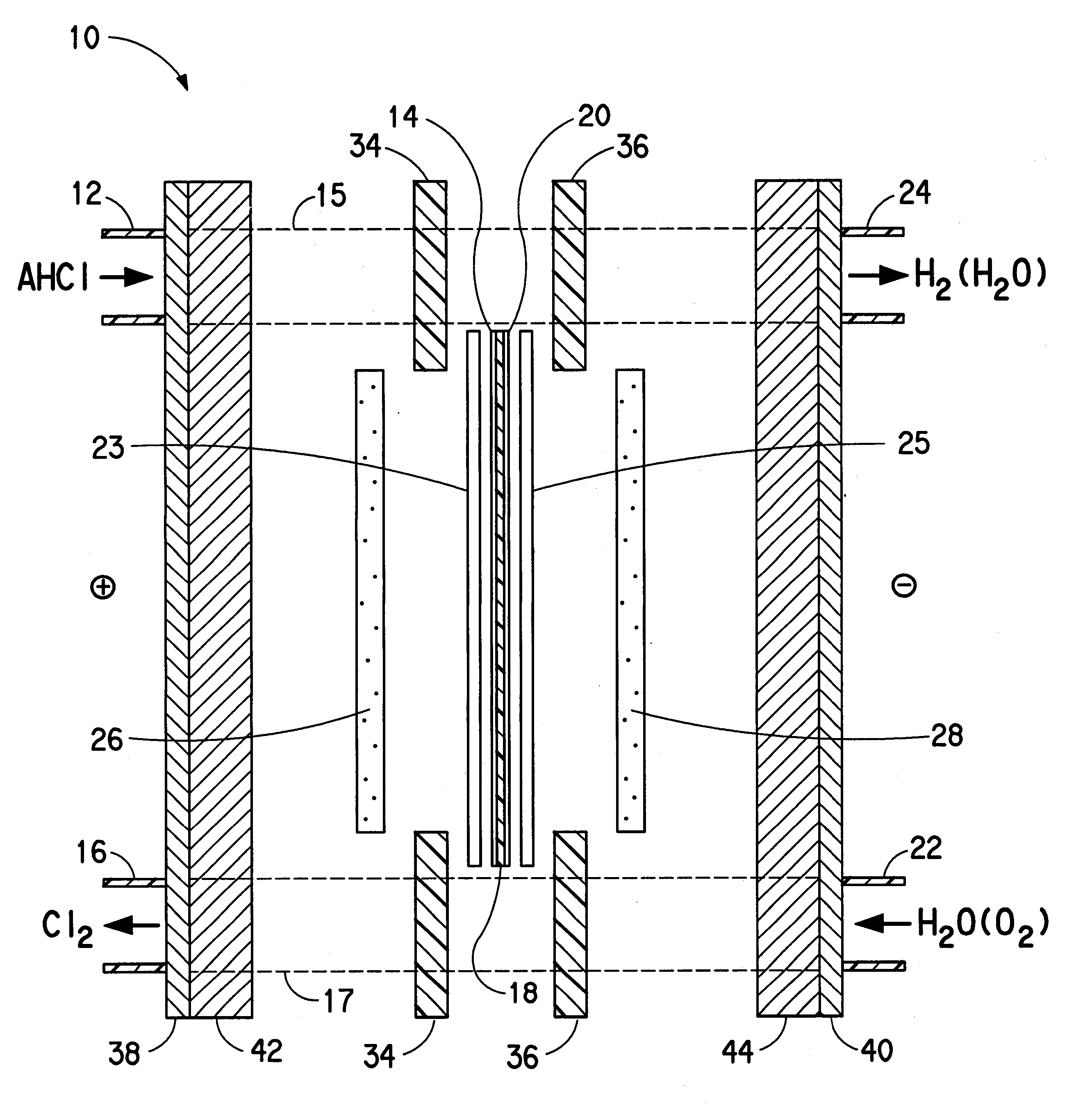
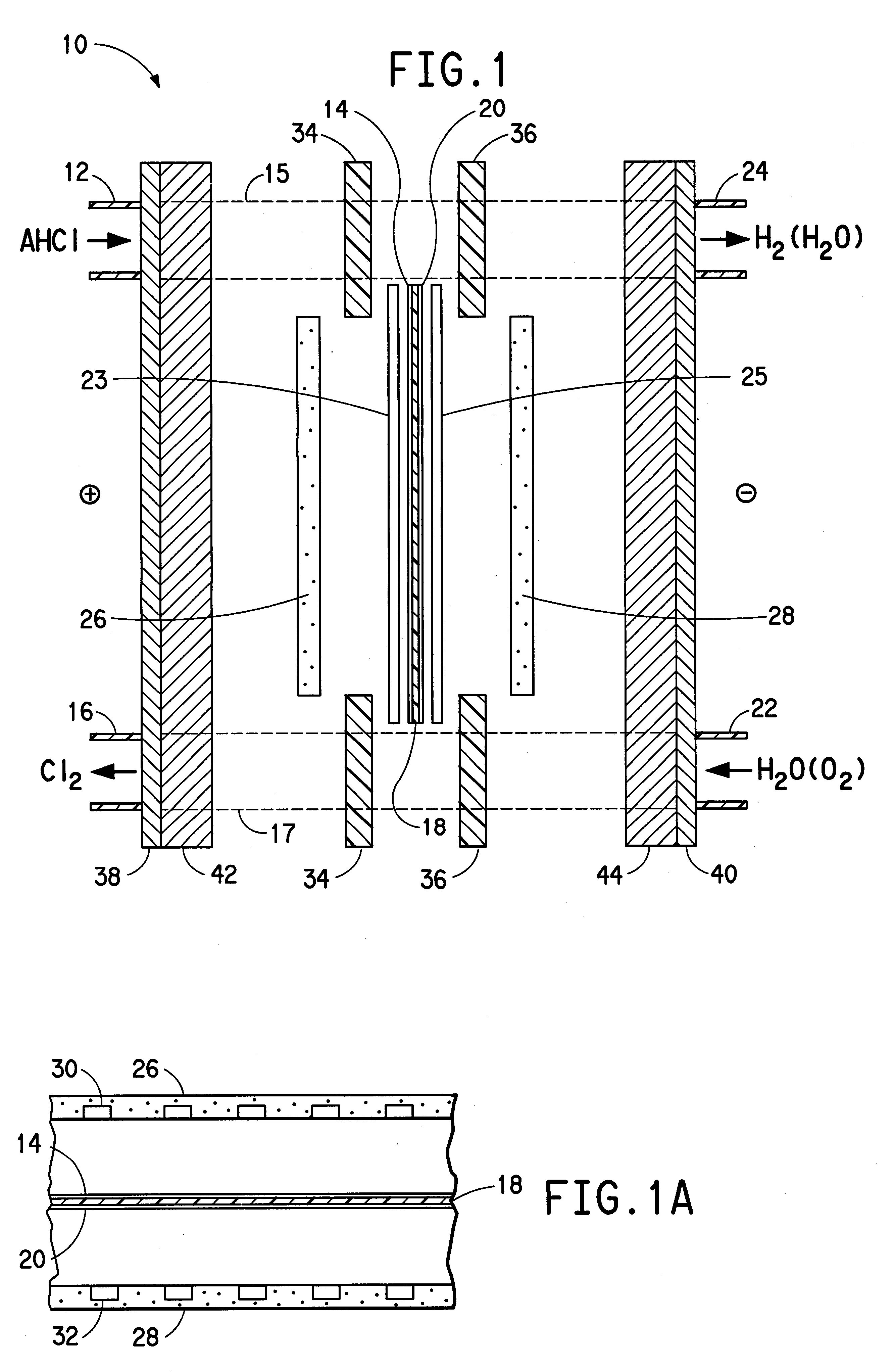
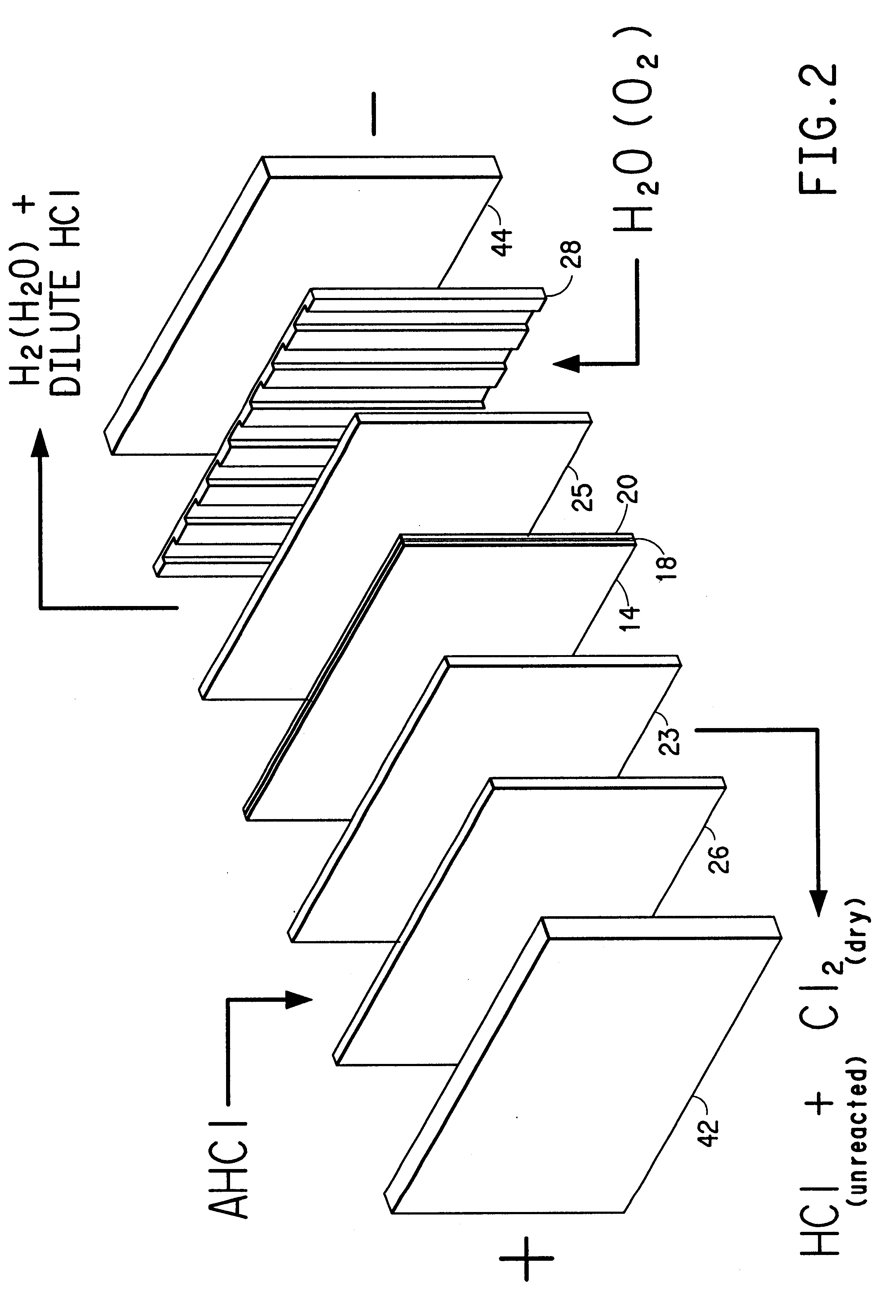
Water is delivered to the cathode through cathode-side inlet 22 as shown in FIG. 1 and through the channels in cathode mass flow field 28 to hydrate the membrane and thereby increase the efficiency of proton transport through the membrane. In the first embodiment, the hydrogen which is evolved at the interface between the cathode and the membrane exits via cathode-side outlet 24. The hydrogen bubbles through the water and is not affected by the electrode. Cathode current distributor 44 collects current from cathode 20, along with cathode diffuser 25, and distributes it to cathode bus 40.
second embodiment
In the second embodiment, a voltage is applied to the anode and the cathode so that the anode is at a higher potential than the cathode, and current flows to the anode bus. Anode current distributor 40 collects current from the anode bus and distributes it, along with anode diffuser 23, to the anode by electronic conduction. Molecules of essentially anhydrous hydrogen chloride are fed to anode-side inlet 14 and are transported through channels of anode mass flow field 26 to the surface of anode 12. An oxygen-containing gas, such as oxygen (O.sub.2 (g)), air or oxygen-enriched air (i.e., greater than 21 mol % oxygen in nitrogen) is introduced through cathode-side inlet 22 and through the channels formed in cathode mass flow field 28. Although air is cheaper to use, cell performance is enhanced when enriched air or oxygen is used. This cathode feed gas may be humidified to aid in the control of moisture in the membrane. Molecules of the hydrogen chloride (HCl(g)) are oxidized under th...
example
Preparation of the Coating Formulation
The coating formulation for an MEA was prepared by adding 15 g of a 50 m.sup.2 / g ruthenium dioxide (RuO.sub.2) catalyst, P-2450, commercially available from Colonial Metals, Inc. of Elkton, Md., to an empty flask which had been purged with dry nitrogen. After additional purging with nitrogen, 200 g of a solution containing a binder polymer, 2.5 wt. % copolymer polymerized from tetrafluoroethylene (TFE) and a vinyl ether which is represented by the formula CF.sub.2.dbd.CF--O--CF.sub.2 CF(CF.sub.3)--O--CF.sub.2 CF.sub.2 SO.sub.2 F dissolved in the solvent FC-40, was added to a flask with constant stirring. The ratio of the catalyst particles to the binder polymer was 3:1.
After the catalyst was fully suspended, the mixture was transferred to a laboratory ball mill and was ground overnight. After the grinding, the particle size of the ruthenium dioxide in the mixture was, on the average, about 5.mu., with many particles being less than 2.mu.. the m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| voltage | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com