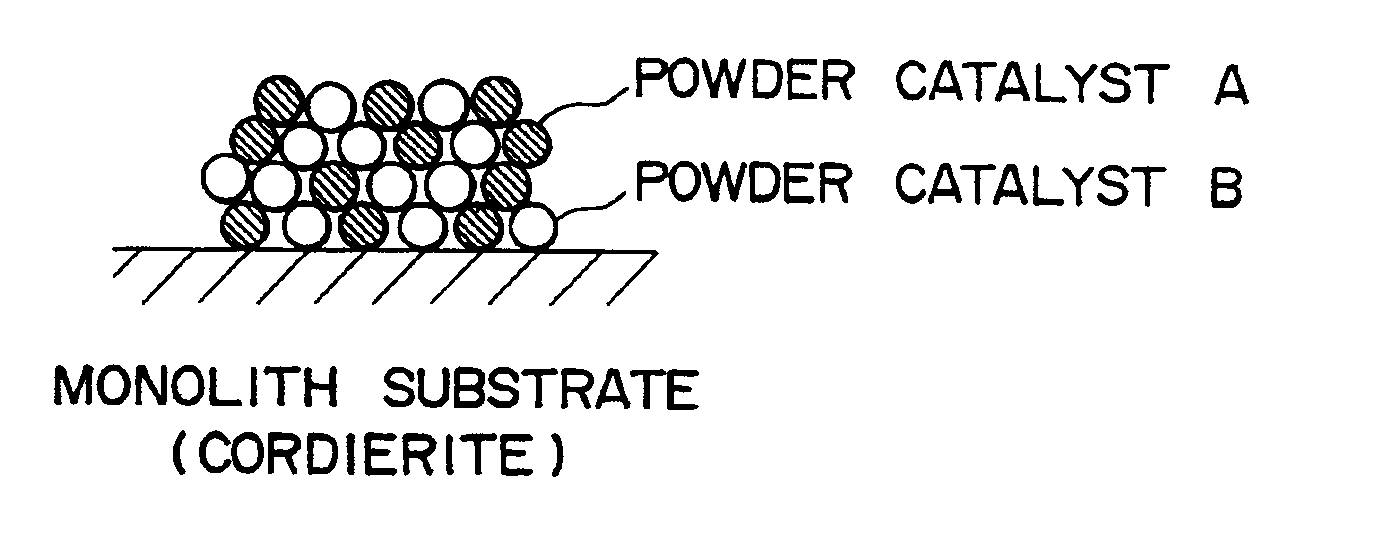
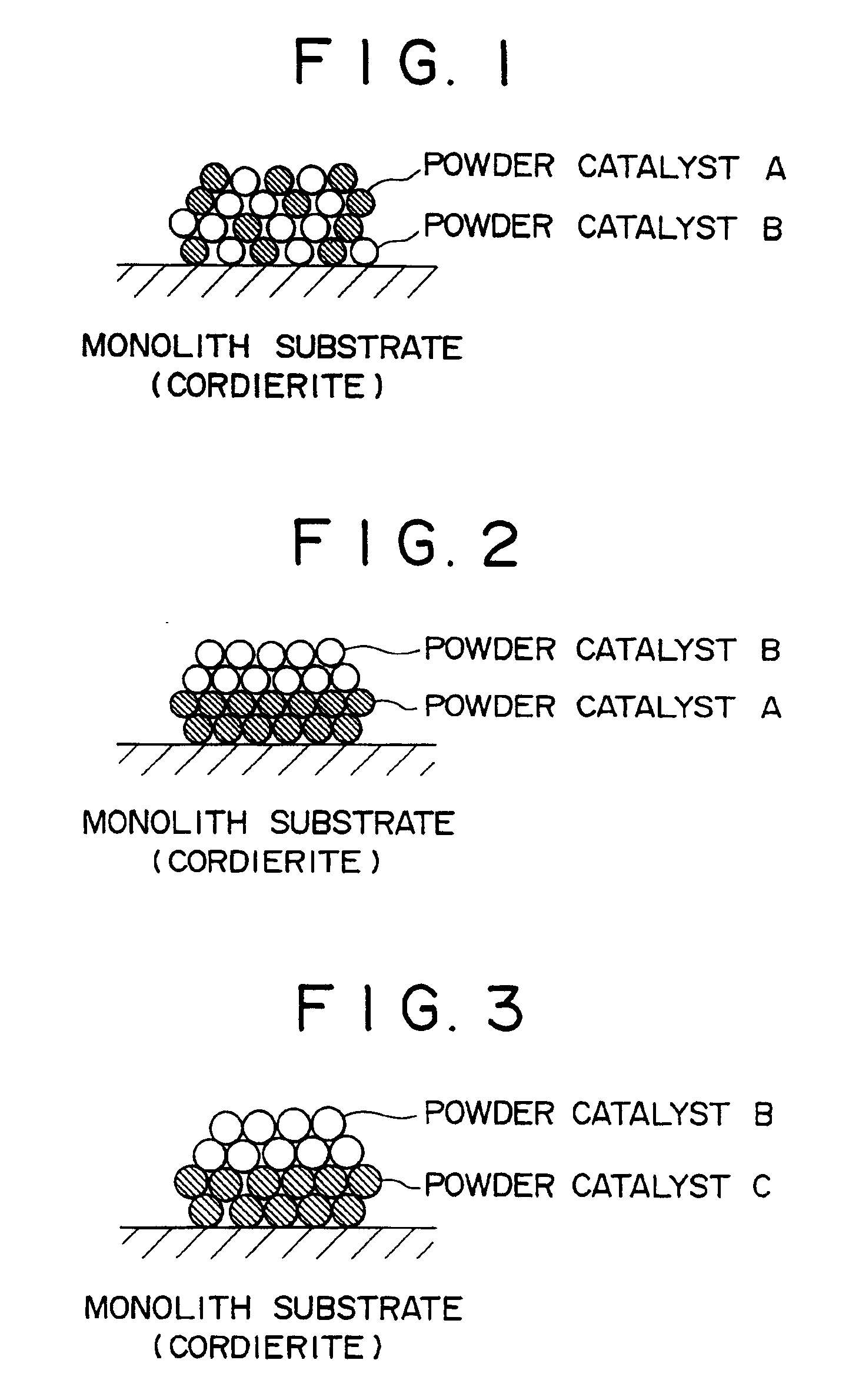
Ammonia decomposition catalysts
a technology of ammonia decomposition and catalyst, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalyst, separation process, etc., can solve the problems of large ammonia release into the atmosphere, large amount of ammonia emitted from various chemical plants, etc., and achieve high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experiment 1
[0047]Ammonia decomposition test was conducted using honeycomb catalysts 1 to 29. Honeycomb catalysts 1 to 29 consisting of 144 cells in the dimension of 15×15×60 mm were placed in a reaction tube, to which ammonia gas having the composition shown below was supplied at SV=16300h−1 and at the flow rate of 5.54Nm3 / m2 to examine ammonia decomposition performance at the reaction temperatures of 300° and 400° C. Gas composition:[0048]NH3: 20 ppm[0049]SO2: 20 ppm[0050]CO2: 7%[0051]H2O: 6%[0052]O2: 14.7%[0053]N2: to 100%
[0054]Performance was evaluated by determining the ammonia decomposition ratio at the initial state of the reaction, NOx (NO, NO2, N2O) production ratio, and SO2 oxidation ratio.
[0055]The ammonia decomposition ratio and NOx production ratio were calculated according to the formulae shown below.[0056]Ammonia decomposition ratio (%)[0057] =[(Inlet NH3−Outlet NH3) / (Inlet NH3)]×100[0058]NOx production ratio (%)=[0059] [(Outlet (N2O×2+NO+NO2)) / (Inlet NH3)]×100[0060]SO2 oxidation...
experiment 2
[0064]Using honeycomb catalysts 1 to 29, the gas was supplied for a long period in the condition similar as in Experiment 1 to conduct durability evaluation test. The results obtained indicated that after the gas supply for 1000 hours in the condition described above the ammonia decomposition ratio, NOx production ratio and SO2 oxidation ratio equivalent to those shown in Table 4 were maintained, ensuring that the catalysts had excellent durability.
[0065]Preparation of catalysts belonging to catalyst A of the second aspect
Preparation of powder catalyst 1
[0066]100 g of crystalline silicate 16f type H described above was immersed in the aqueous solution of iridium chloride (1 g of IrCl in 100 cc of water), kneaded thoroughly, and then evaporated to dryness at 200° C. After purging with nitrogen for 12 hours at 500° C., powder catalyst 1 which is classified to catalyst A was obtained.
Preparation of powder catalysts 2 to 15
[0067]Crystalline silicate 2 to 15 of type H listed above were i...
experimental example 3
[0082]Honeycomb catalysts 30 to 95 were subjected to the ammonia decomposition test similarly as in Example 1. The results are shown in Tables 7 and 8.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com