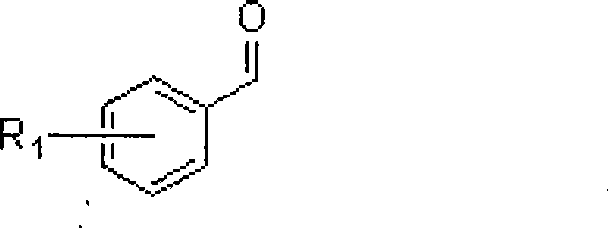
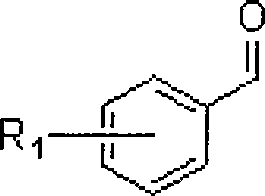Method for synthesizing 3-aryl allyl group ether
A kind of technology of aryl allyl ether and synthesis method, which is applied in ether preparation, organic chemistry, etc., can solve the problems of non-recyclable metal, harm to human body and environment, instability of allyl tin, etc., and achieve moderate activity and low price Inexpensive, easy to operate and convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Ferric chloride catalyzes the reaction of benzaldehyde with allyltriethoxysilane to prepare 3-phenylallyl ethyl ether.
[0018] Put a 10ml glass bottle with a rubber stopper on the 85-2 type constant temperature magnetic stirrer, add 2ml of nitromethane, 0.106g (1mmol) benzaldehyde, 0.224g (1.1mmol) allyltriethoxysilane, quickly Weigh 0.0162g (0.1mmol) of ferric trichloride into the reaction bottle, and cover with a rubber stopper. Then stir at room temperature for 3 hours to react. Then extracted three times with 1:1 petroleum ether / diethyl ether (15ml×3), combined the organic phases, dried over anhydrous sodium sulfate, and evaporated the solvent under reduced pressure to obtain 1.67g of oily liquid product with a yield of 95%. m / z176(M + )135(100)107(79)79(63) 1 H NMR (400MHz, CDCl 3 ) 7.30 (m 5H) 5.77 (m 1H) 5.34 (m 2H) 4.26 (t J = 8.0Hz 1H) 3.36 (m 2H) 2.57 (m 1H) 2.40 (m 1H) 1.18 (t J = 8.0Hz 3H) 13 C NMR (100MHz, CDCl 3 ) 142.26 134.84 128.12 127.28 126.48 ...
Embodiment 2
[0020] Ferric chloride catalyzes the reaction of o-chlorobenzaldehyde and allyl triethoxysilane to prepare 3-(1-chlorophenyl) allyl ethyl ether.
[0021] Put a 10ml glass bottle with a rubber stopper on the 85-2 type constant temperature magnetic stirrer, add 2ml of nitromethane, 0.141g (1mmol) o-chlorobenzaldehyde, 0.224g (1.1mmol) allyltriethoxysilane , quickly weighed 0.0162g (0.1mmol) ferric chloride into the reaction bottle, and covered with a rubber stopper. Then it was stirred for 3 hours at normal temperature. Then it was extracted three times with 1:1 petroleum ether / diethyl ether (15mlx3), the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain 0.202 g of an oily liquid product with a yield of 96%. m / z210(M + )171(28)169(84)141(100)113(30) 1 H NMR (400MHz, CDCl 3 )7.49(m 1H)7.30(m 2H)7.20(m 1H)5.87(m 1H)5.05(m 2H)3.87(m 1H)3.38(m 2H)2.46(m 2H)1.19(t J=8.0Hz 3H) 13 C NMR (100MHz, CDCl ...
Embodiment 3
[0023] Indium trichloride catalyzes the reaction of p-bromobenzaldehyde with allyl triethoxysilane to prepare 3-(3-bromophenyl) allyl ethyl ether.
[0024] Put a 10ml glass bottle with a rubber stopper on the 85-2 type constant temperature magnetic stirrer, add 2ml of dichloromethane, 0.185g (1mmol) p-bromobenzaldehyde, 0.224g (1.1mmol) allyltriethoxysilane , quickly weighed 0.0221g (0.1mmol) indium trichloride, 0.163g (1.5mmol) trimethylchlorosilane and added to the bottle. Then it was stirred for 3 hours at normal temperature. Then extracted three times with 1:1 petroleum ether / diethyl ether (15mlx3), combined the organic phases, dried over anhydrous sodium sulfate, and evaporated the solvent under reduced pressure to obtain 0.225g of oily liquid product with a yield of 96%. m / z255(M + )215(92)213(100)187(75)185(89)157(32)77(62) 1 H NMR (400MHz, CDCl 3 ) 7.46 (d J = 8.0Hz 2H) 7.18 (d J = 8.0Hz 2H) 5.74 (m 1H) 5.02 (m 2H) 4.23 (t J = 6.0Hz 1H) 3.34 (m 2H) 2.54 (m 1H) 2.35...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com