Oral preparation for reducing brinolase, preparation method and uses thereof
An oral preparation, defibrase technology, applied in the field of defibrase oral preparations, can solve problems such as inconvenient use, low bioavailability, and patient pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] The preparation method of enteric-coated tablet generally includes steps such as granulation, tabletting, and coating.
[0064] The preparation method of enteric-coated capsules usually includes the steps of granulating and encapsulating.
[0065] The preparation method of enteric-coated pellets usually includes: the preparation methods of pellets include coating pot method, centrifugal layering method, spheroid granulation method, emulsification method, extrusion spheronization method, fluidized bed granulation coating, oscillating drop There are differences in the specific operation steps in methods such as preparation methods.
[0066] In another preferred example, the obtained mixture is prepared into microcapsules, and coated into enteric-coated tablets after tableting, or the microcapsules are filled into enteric-coated capsule shells to make enteric-coated capsules, or the microcapsules are made into Enteric-coated pellets.
[0067] Spherical pellet granulator ...
Embodiment 1
[0105] Embodiment 1, preparation of defibrase enteric-coated capsules
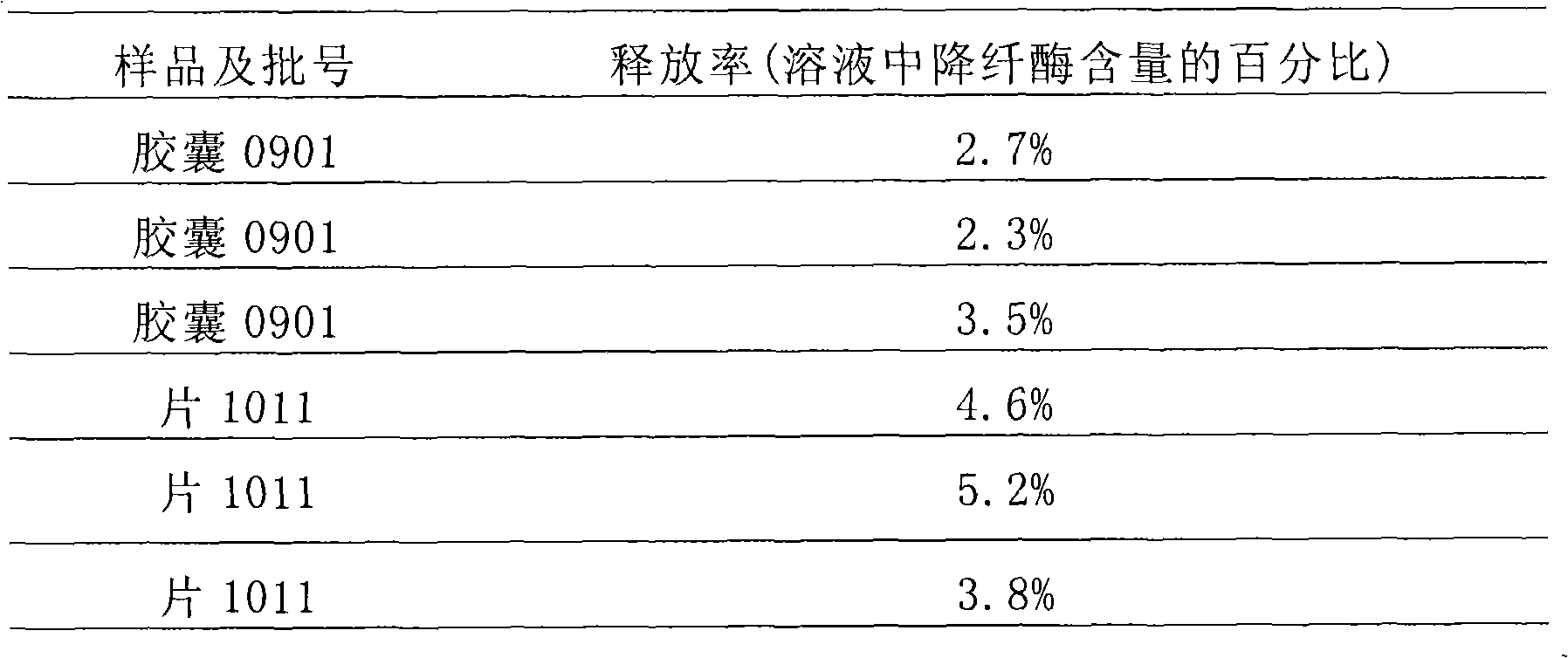
[0106] Take defibrase 0.2g (protein weight, specific activity is 2000U / mg protein) and fully mix with carboxymethyl cellulose salt 15g, low-substituted hydroxypropyl cellulose 15g, cross-linked polyvinylpyrrolidone 10g, gelatin 5g, etc., to prepare Form microcapsules, then fully mix the microcapsules with 5 g of microcrystalline cellulose, 25 g of starch, and talcum powder, pack into No. 3 enteric-coated capsules, and prepare 1000 enteric-coated capsules, each capsule containing 0.2 mg of defibrase.
[0107] In this embodiment, multiple batches of capsules were prepared, and the batch numbers 0901, 0902, and 0903 were randomly selected for testing.
Embodiment 2
[0108] Embodiment 2, preparation of defibrase enteric-coated tablets
[0109] Take defibrase 0.2g (specific activity is about 1500U / mg protein) and carboxymethyl cellulose salt 15g, low-substituted hydroxypropyl cellulose 10g, cross-linked polyvinylpyrrolidone 4g, gelatin 5g, etc., fully mix, granulate, Freeze-dried, coated with cellulose acetate phthalate, passed through a 18-24 mesh sieve, and then mixed with 5 g of microcrystalline cellulose, 25 g of starch, 4 g of cross-linked polyvinylpyrrolidone, 4 g of cross-linked sodium carboxymethyl cellulose, Low-substituted-hydroxypropyl cellulose 5g, appropriate amount of magnesium stearate and micropowder silica gel, mixed and pressed into 1000 tablets. Each tablet contains 0.2mg defibrase.
[0110] In this example, multiple batches of enteric-coated tablets were produced, among which batch numbers 1011, 1012, and 1013 were randomly selected for testing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com