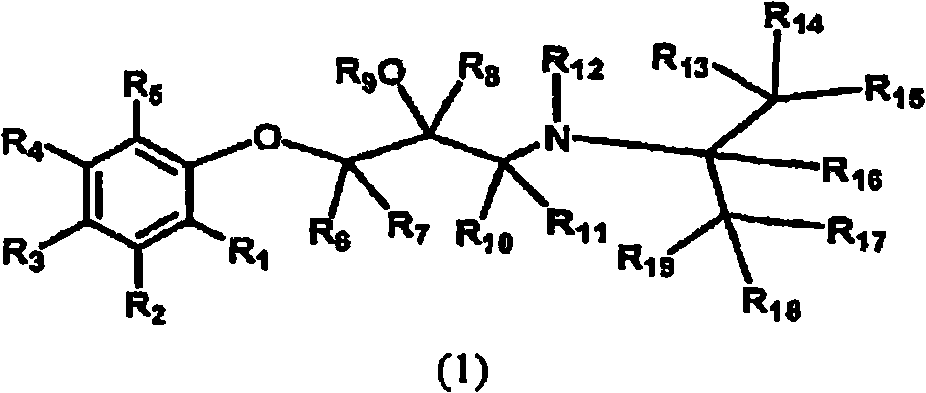
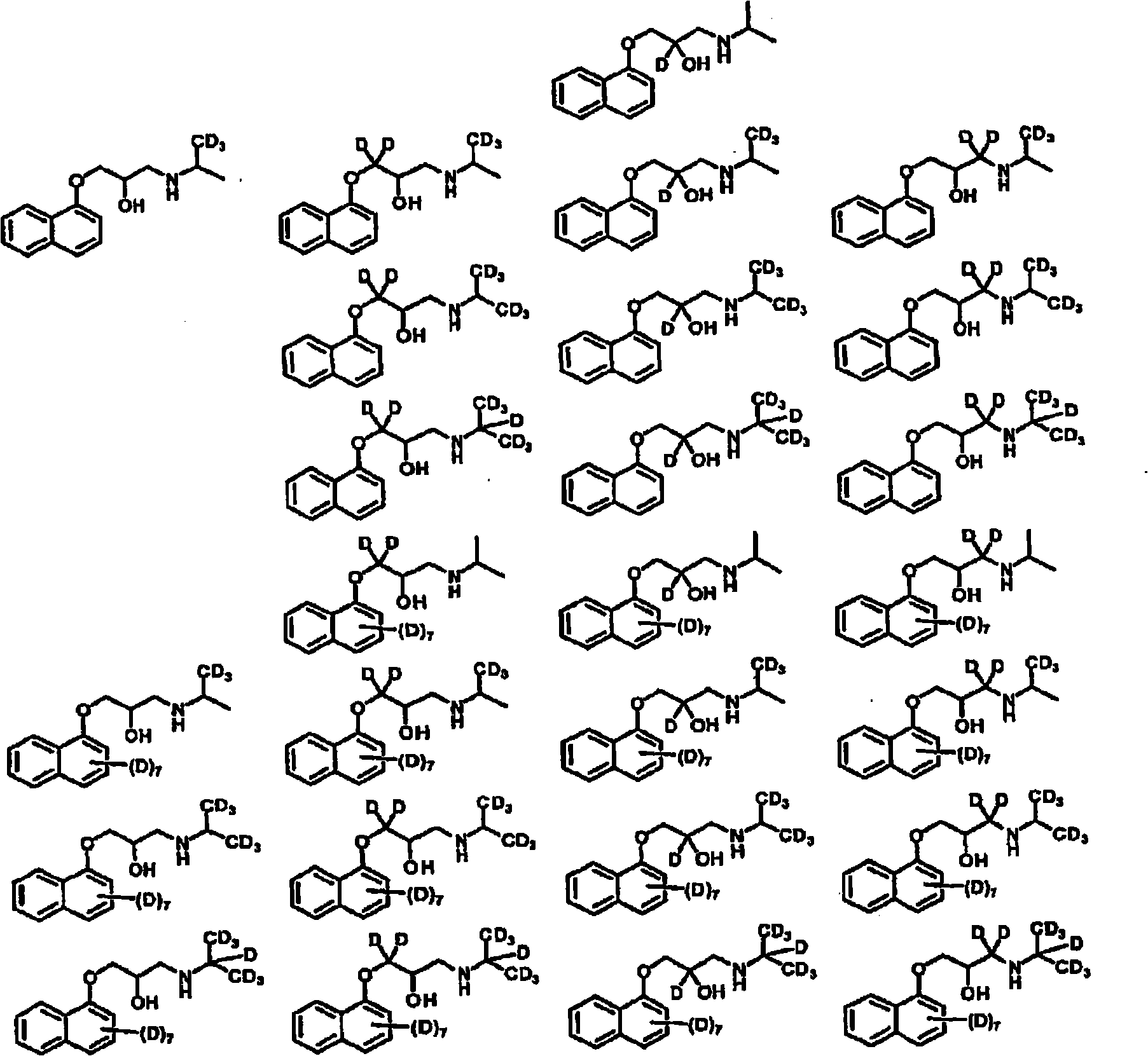
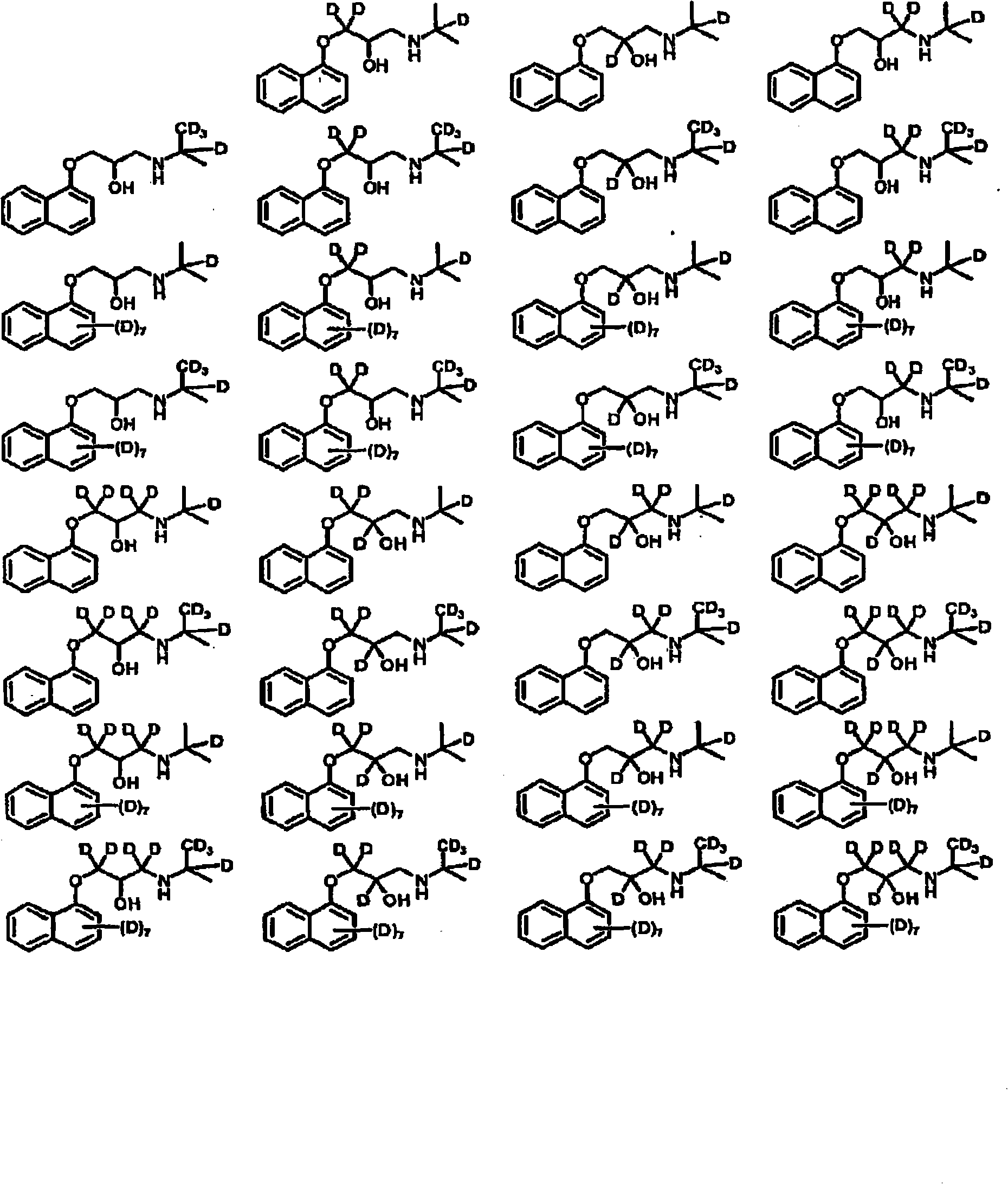
Deuterated aminoglycidyl compounds
A technology for compounds and mixtures, applied in the field of deuterated aminoglycidyl compounds, can solve problems such as exacerbating differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0191]Acids suitable for use in the preparation of pharmaceutically acceptable salts include, but are not limited to, acetic acid, 2,2-dichloroacetic acid, acylated amino acids, adipic acid, alginic acid, ascorbic acid, L-aspartic acid, Benzenesulfonic acid, benzoic acid, 4-acetamidobenzoic acid, boric acid, (+)-camphoric acid, camphorsulfonic acid, (+)-(1S)-camphor-10-sulfonic acid, capric acid, caproic acid, octanoic acid , cinnamic acid, citric acid, cyclamic acid, cyclamate, dodecylsulfonic acid, ethane-1,2-disulfonic acid, ethanesulfonic acid, 2-hydroxy-ethanesulfonic acid, Formic acid, fumaric acid, galactaric acid, gentisic acid, glucoheptonic acid, D-gluconic acid, D-glucuronic acid, L-glutamic acid, α-oxo-glutaric acid , glycolic acid, hippuric acid, hydrobromic acid, hydrochloric acid, hydroiodic acid, (+)-L-lactic acid, (±)-DL-lactic acid, lactobionic acid, lauric acid, maleic acid, (-)-L-maleic acid acid, malonic acid, (±)-DL-mandelic acid, methanesulfonic acid, n...
Embodiment 1
[0330] 2-(4-Benzyloxyphenyl)-methyl acetate
[0331]
[0332] To a solution of (4-hydroxy-phenyl)-acetic acid methyl ester (30 mmol) in 25 mL of anhydrous dimethylformamide was added potassium carbonate (34 mmol) and 4 mL of benzyl bromide at 0 °C under nitrogen atmosphere (34mmol). The mixture was heated to 60°C for 3 hours. After cooling to ambient temperature, the reaction mixture was poured into water. The organic phase was separated and the aqueous layer was extracted with ethyl acetate. The combined organic layers were washed with brine and dried over magnesium sulfate. After removal of the solvent, the residue was purified by flash column chromatography to give the title compound as an oil. Yield: 83%. 1 H-NMR (CDCl 3 ): δ7.35-7.47(m, 5H); 7.23(d, J=8.8Hz, 2H); 6.96(d, J=8.8Hz, 2H); 5.07(s, 2H); 3.63(s, 3H) ; 3.60(s, 2H).
Embodiment 2
[0334] 2-(4-Benzyloxyphenyl)-ethanol
[0335]
[0336] A solution of 2-(4-benzyloxyphenyl)-acetic acid methyl ester (7.8 mmol) in dry ether (5 mL) was added to lithium aluminum hydride (15.8 mmol) in 25 mL of dry ether at 0 °C in the suspension. The reaction mixture was stirred overnight at ambient temperature, quenched with water and extracted with ethyl acetate. The combined organic layers were dried over sodium sulfate. The solvent was removed under reduced pressure to afford the title compound. Quantitative yield. 1 H-NMR (CDCl 3 ): δ7.28-7.47(m, 5H); 7.17(d, J=8.7Hz, 2H); 6.95(d, J=8.7Hz, 2H); 5.07(s, 2H); 3.85(t, J= 6.6Hz, 2H); 2.84(t, J = 6.6Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com