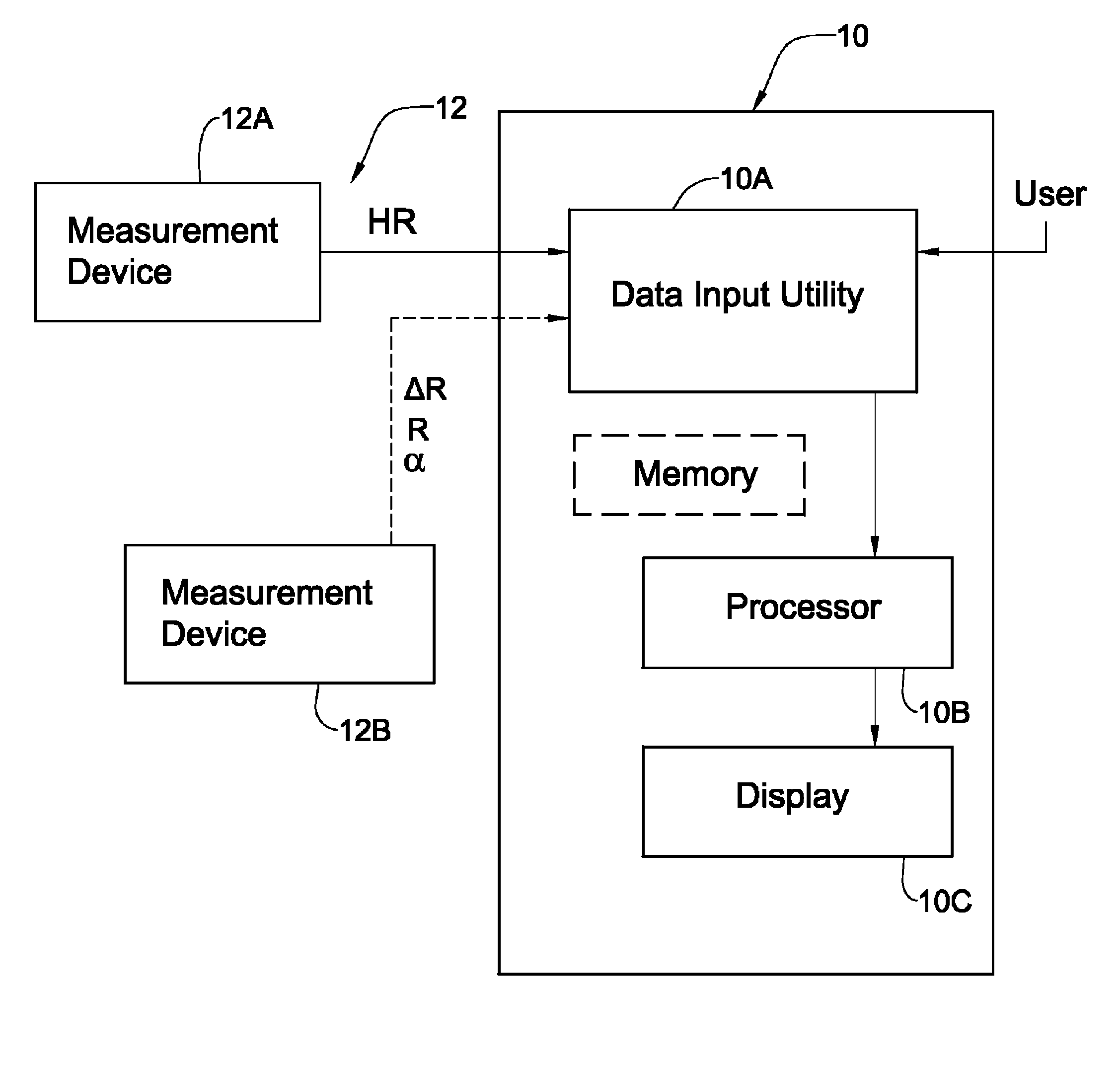
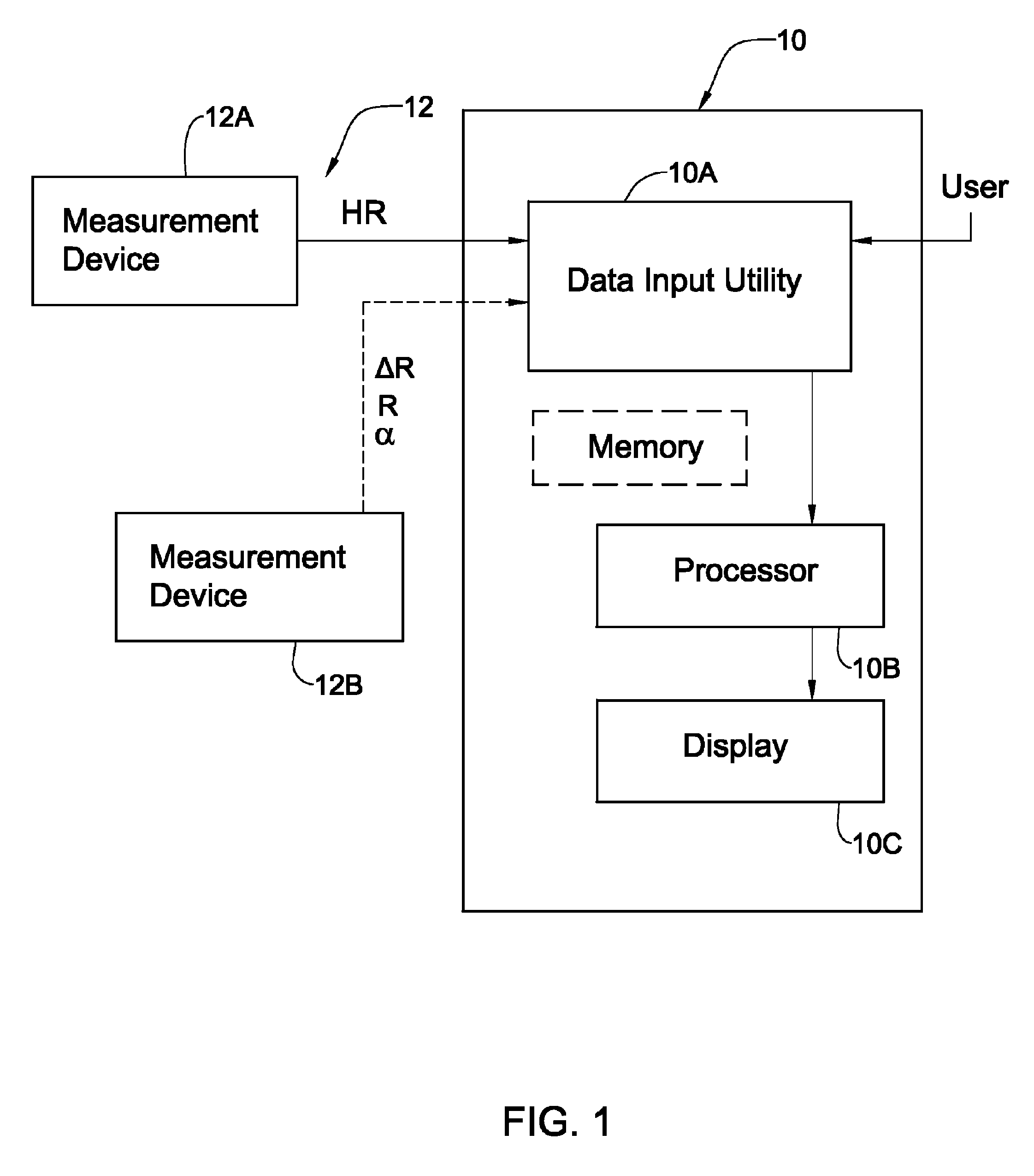
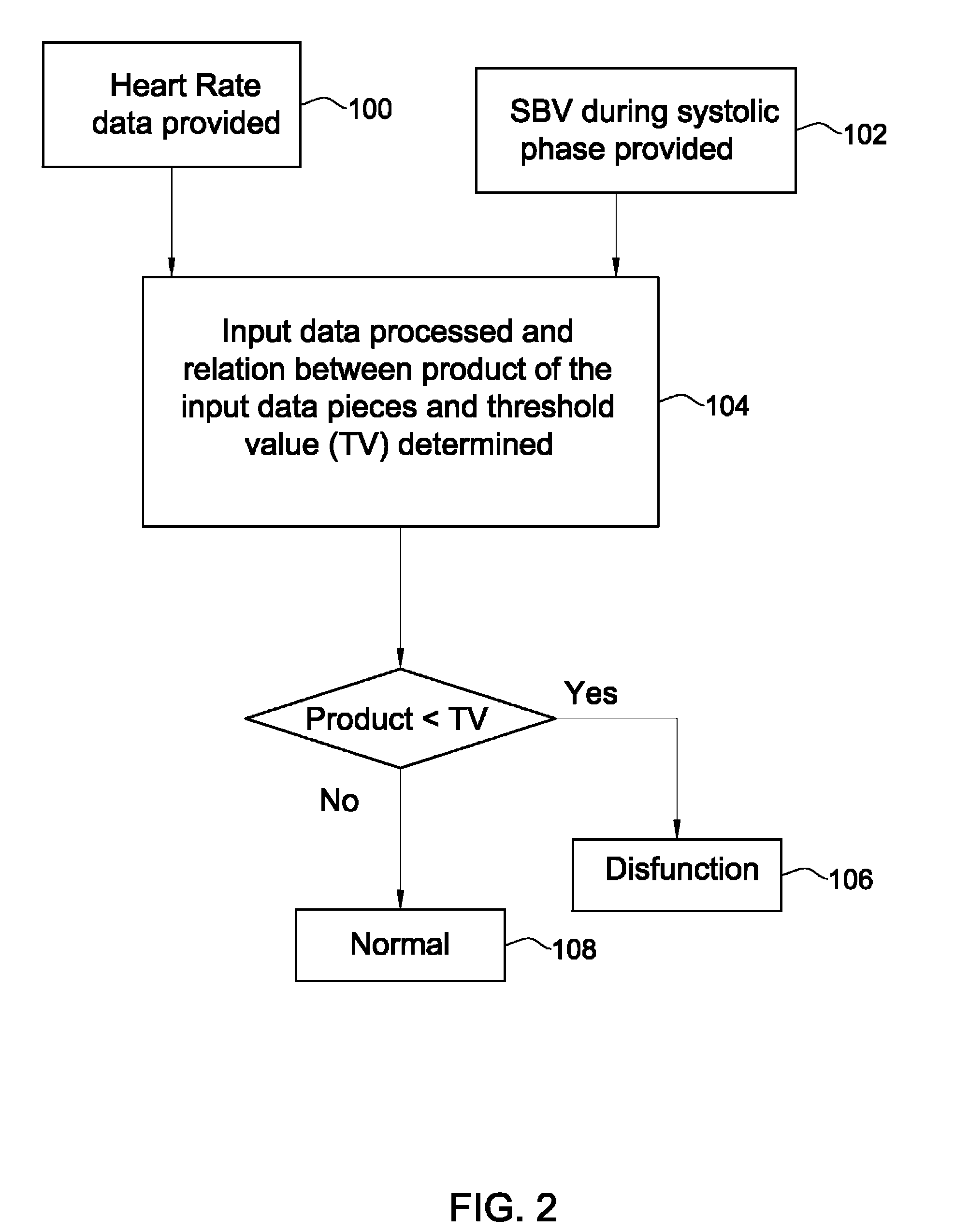
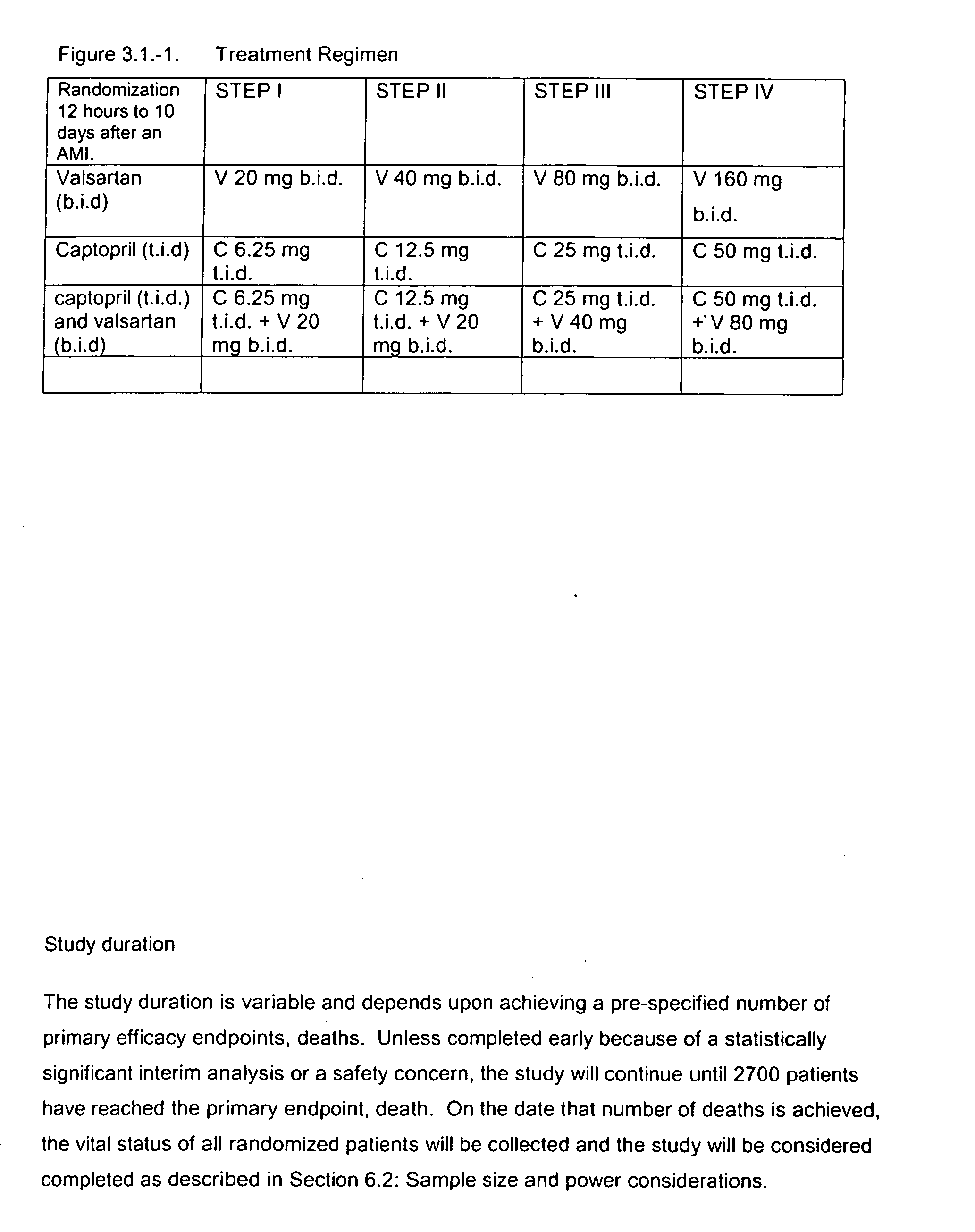
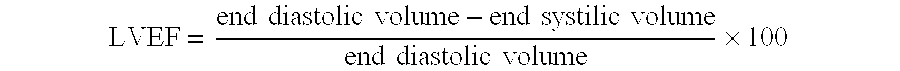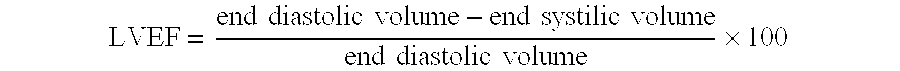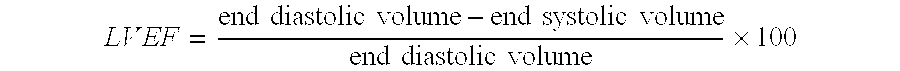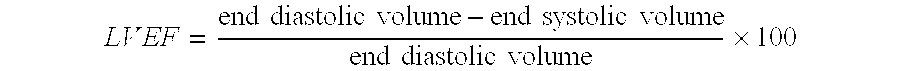Patents
Literature
37 results about "Linksventrikulare funktion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
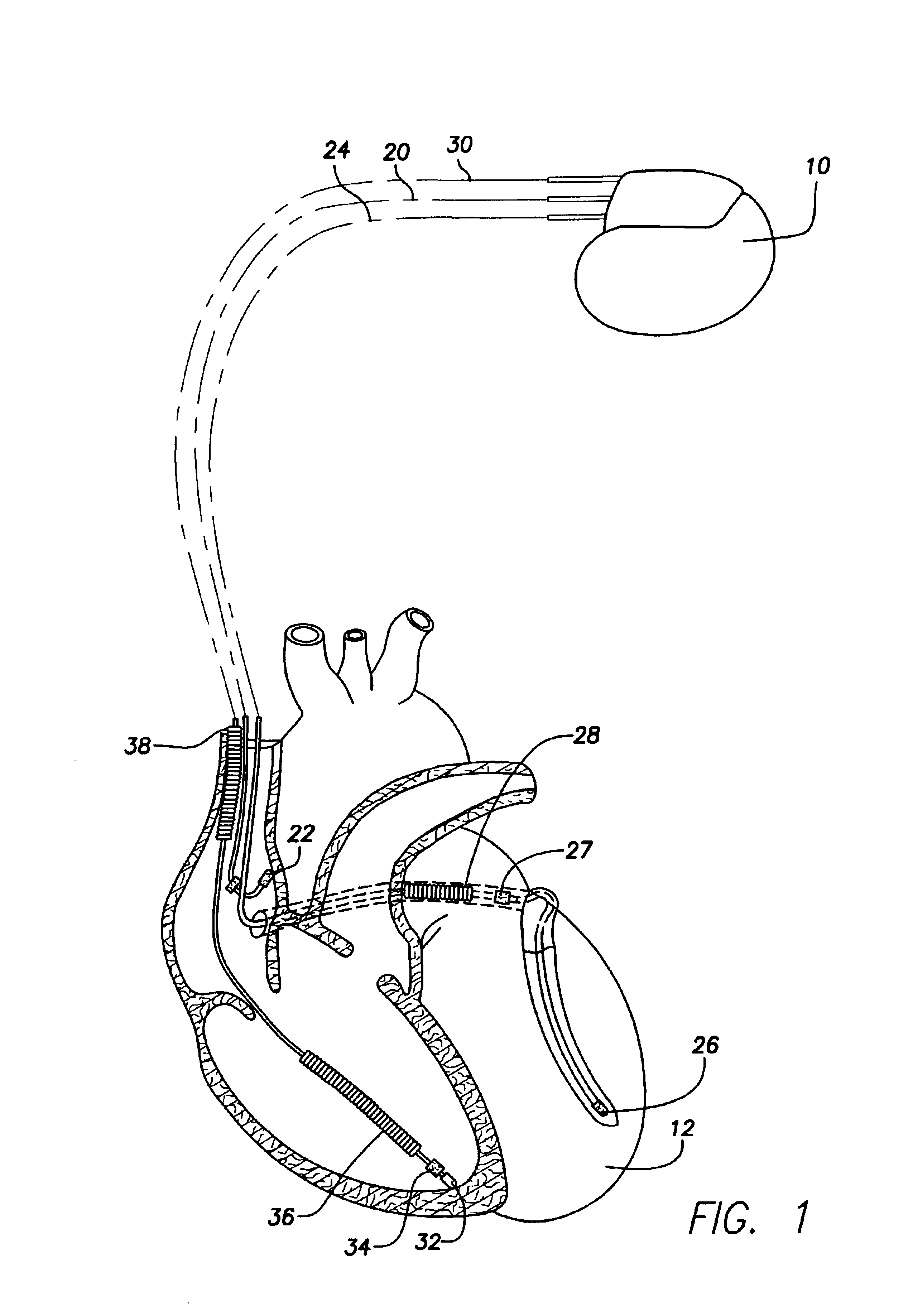
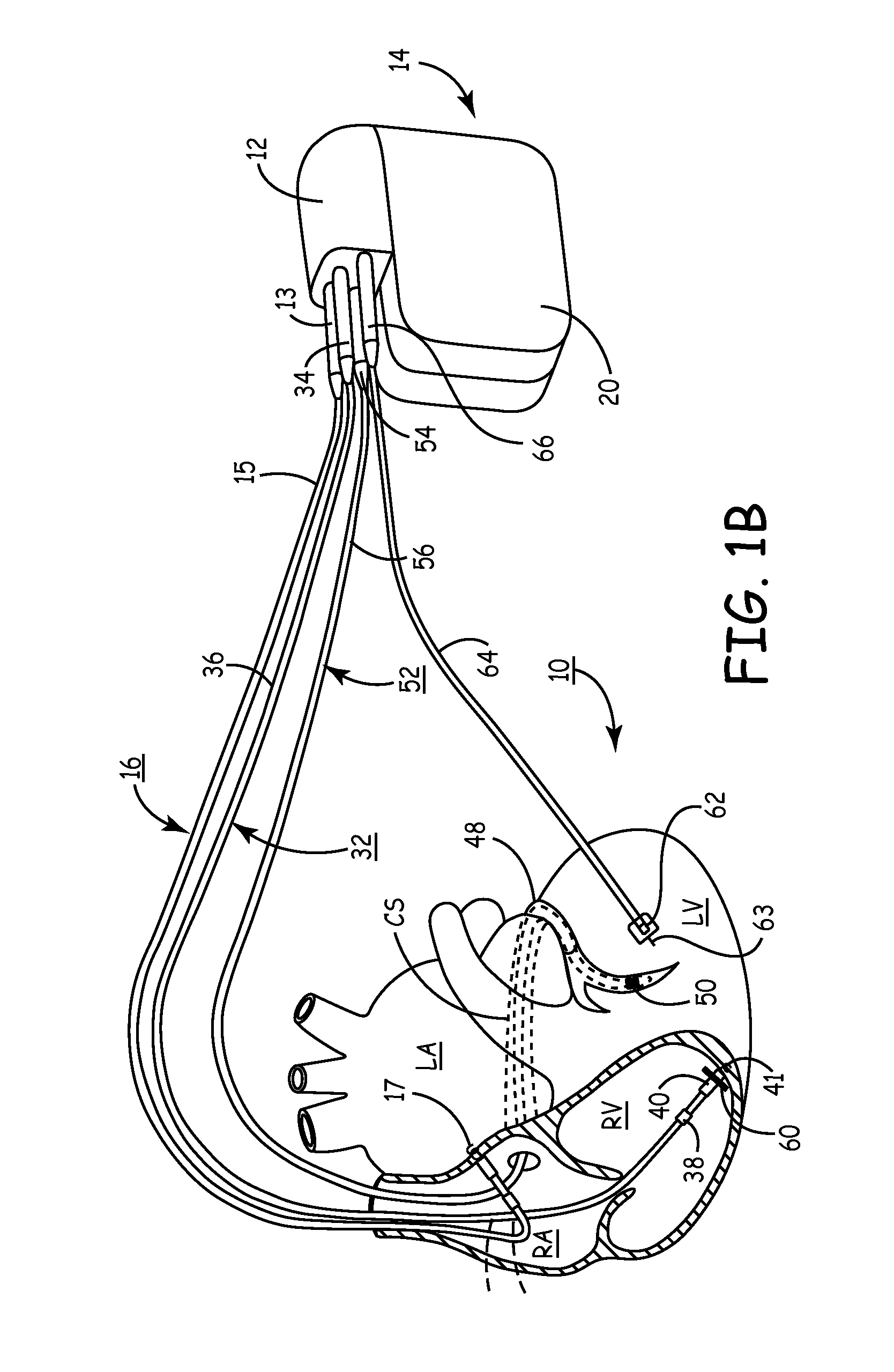
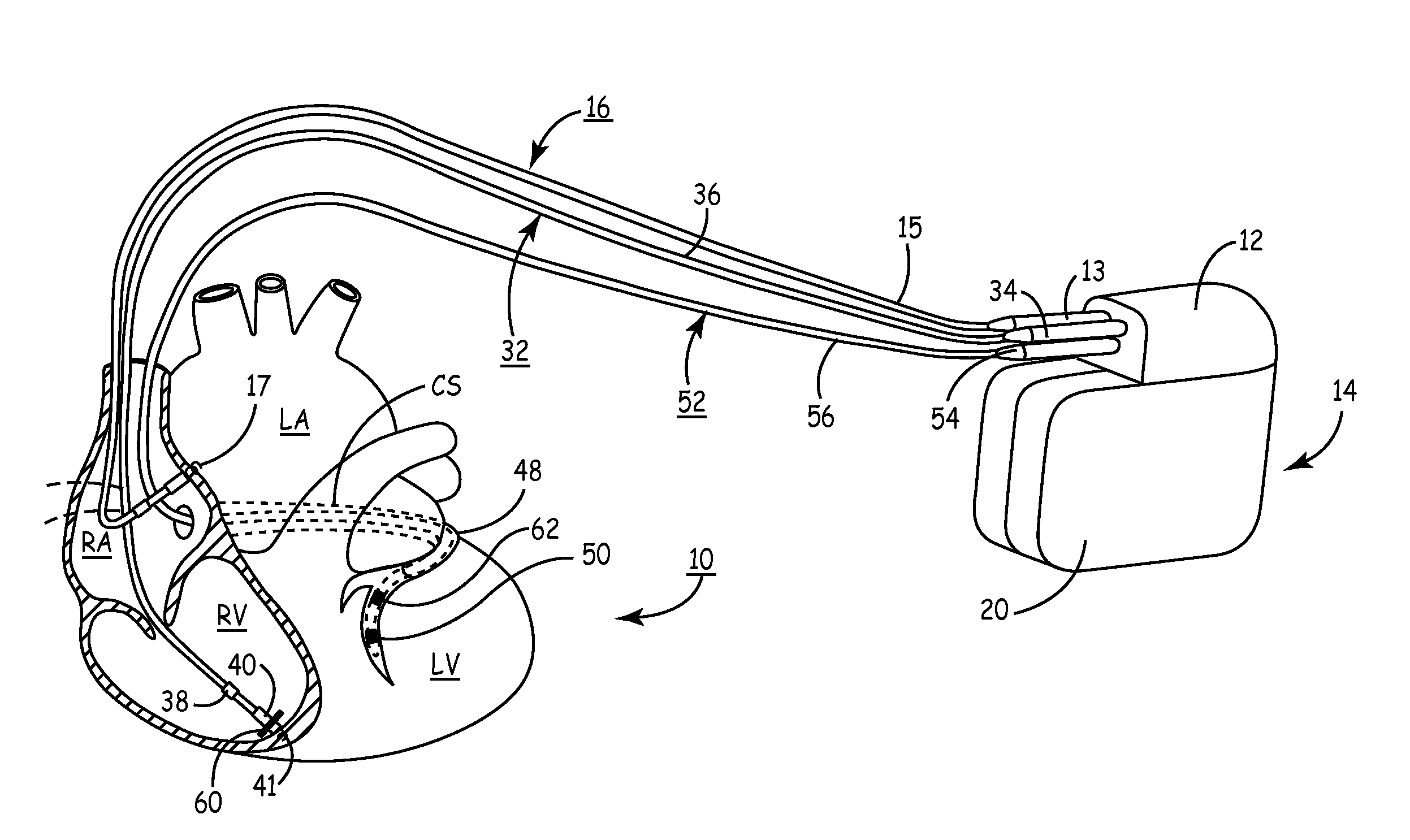
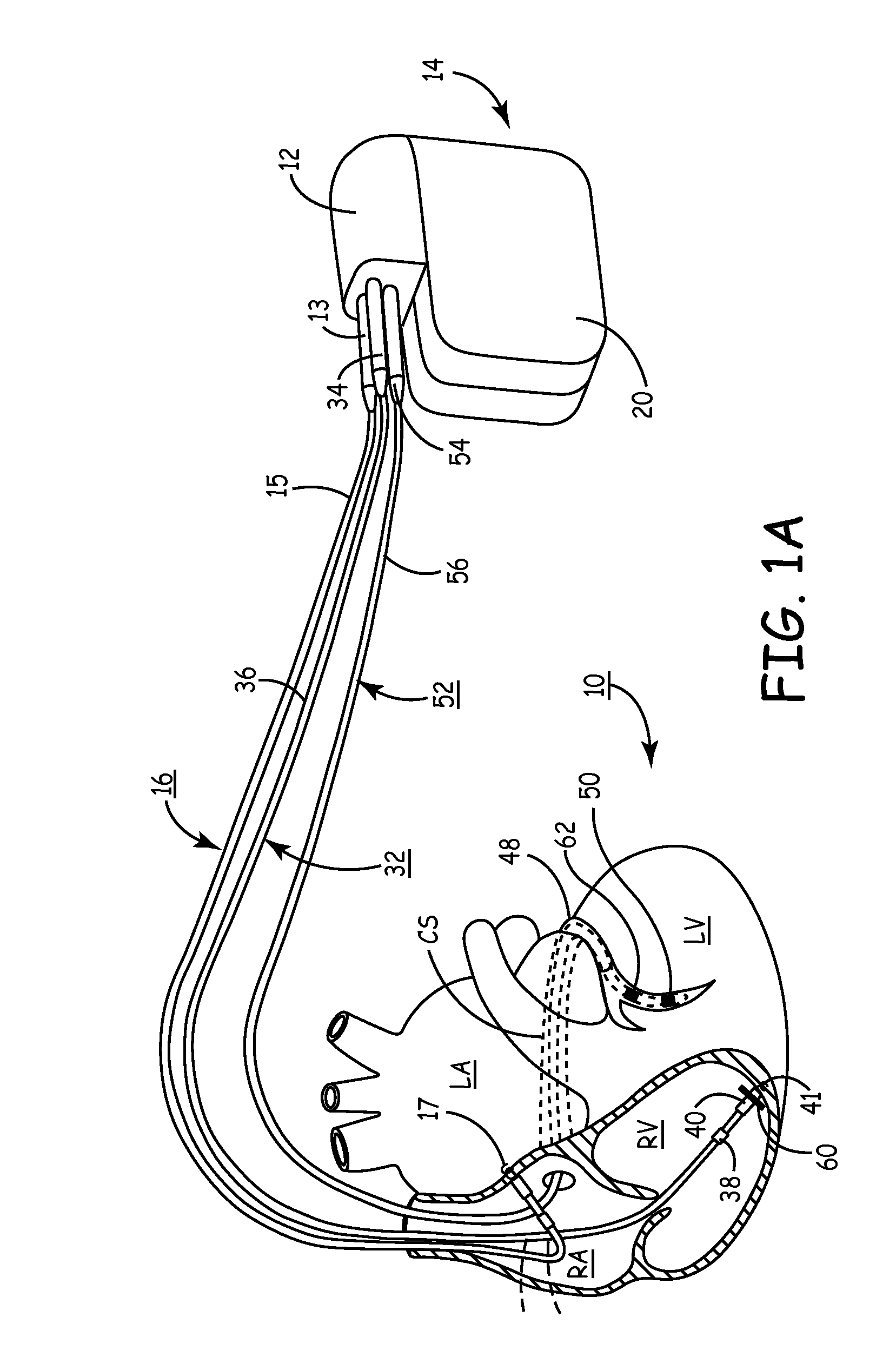
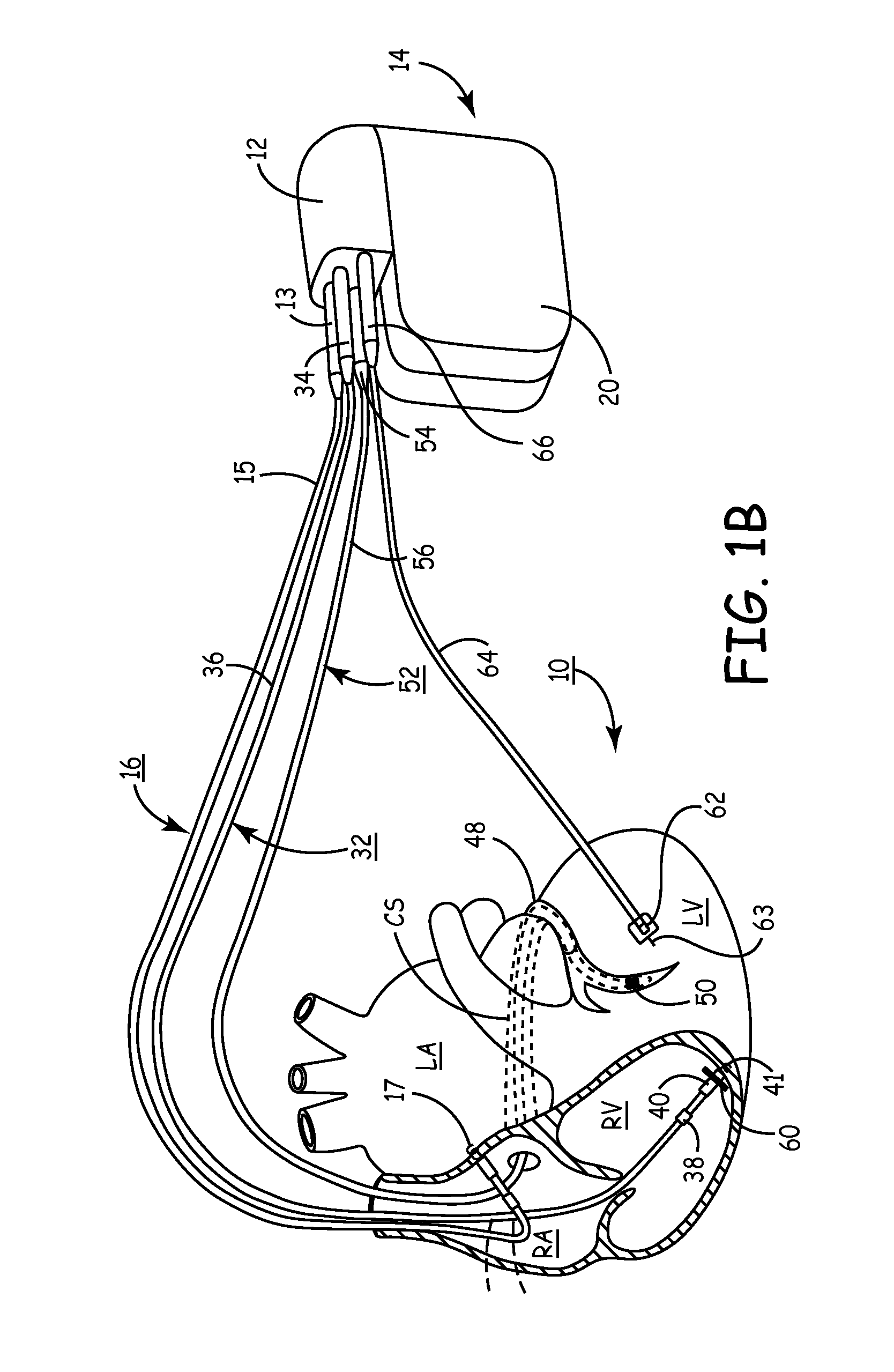
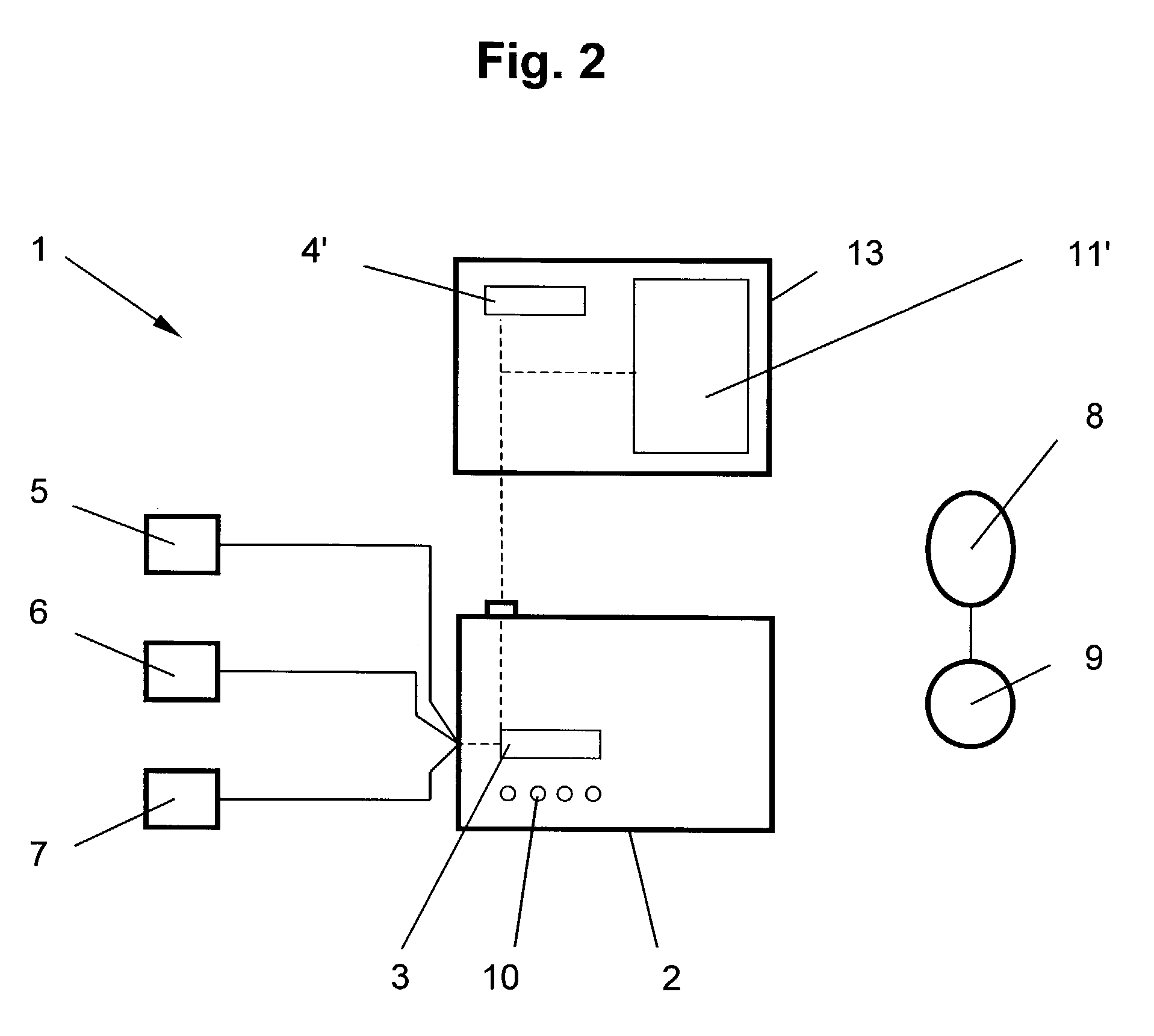
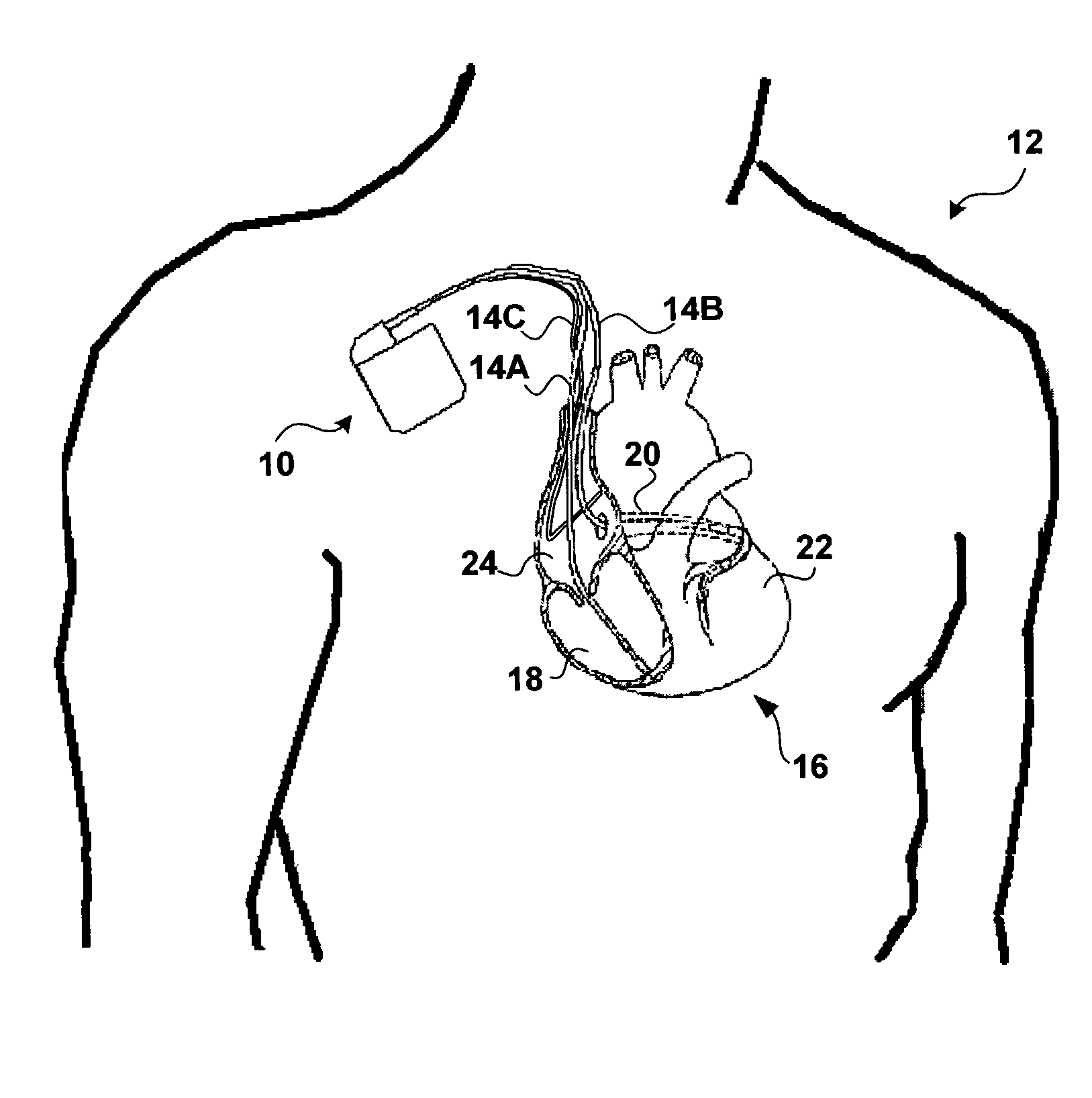
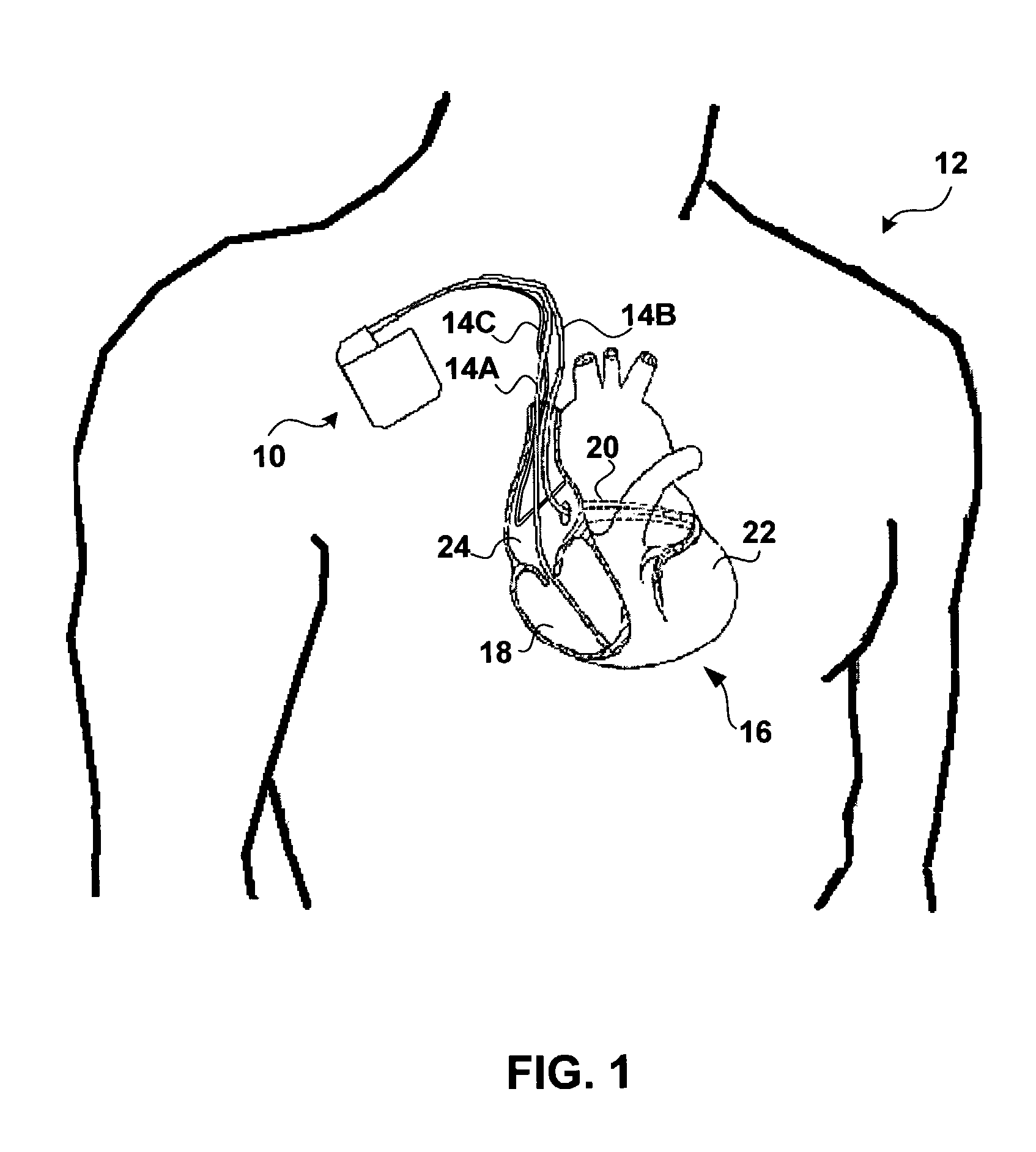
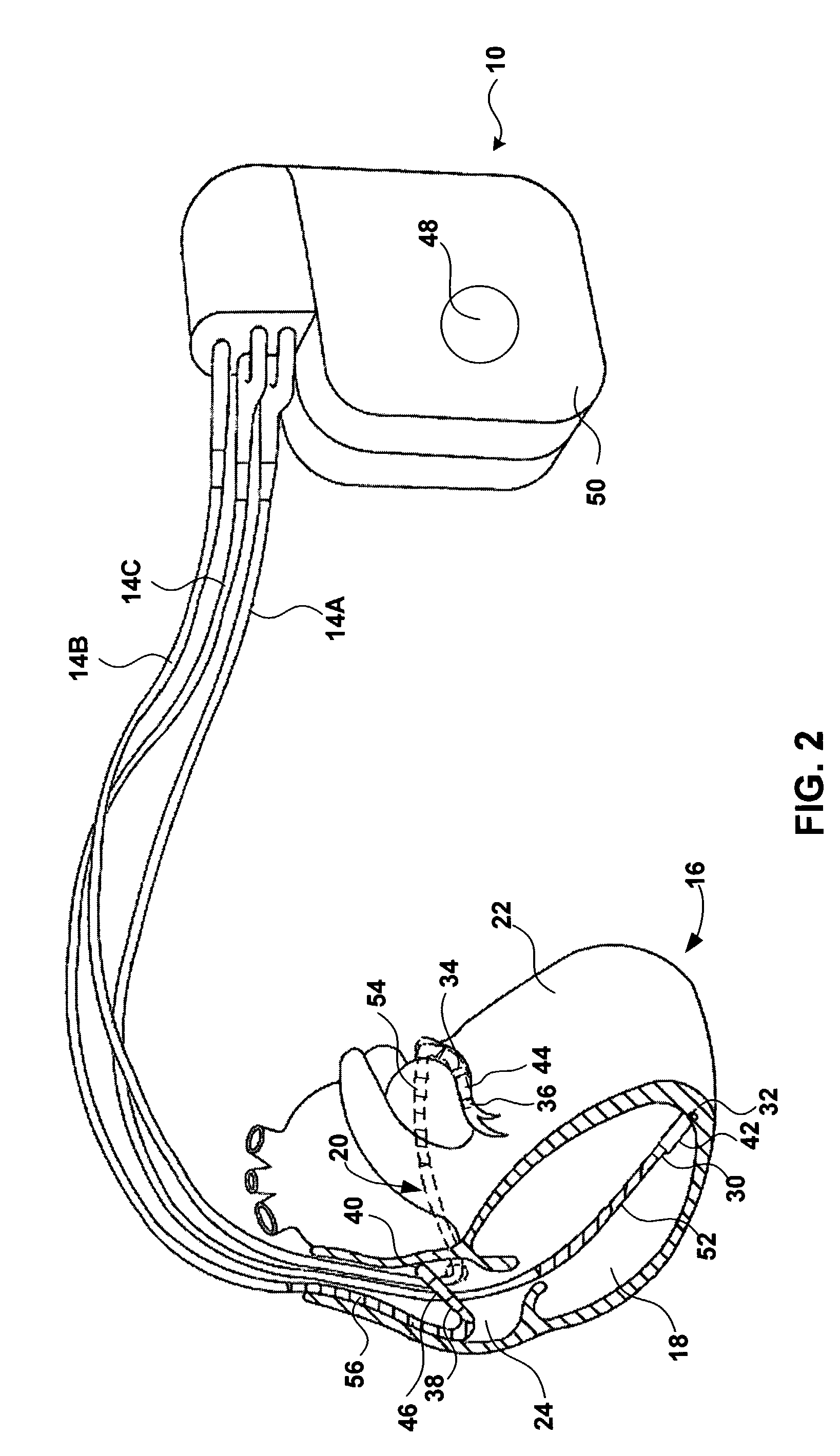
Method and apparatus for assessing left ventricular function and optimizing cardiac pacing intervals based on left ventricular wall motion
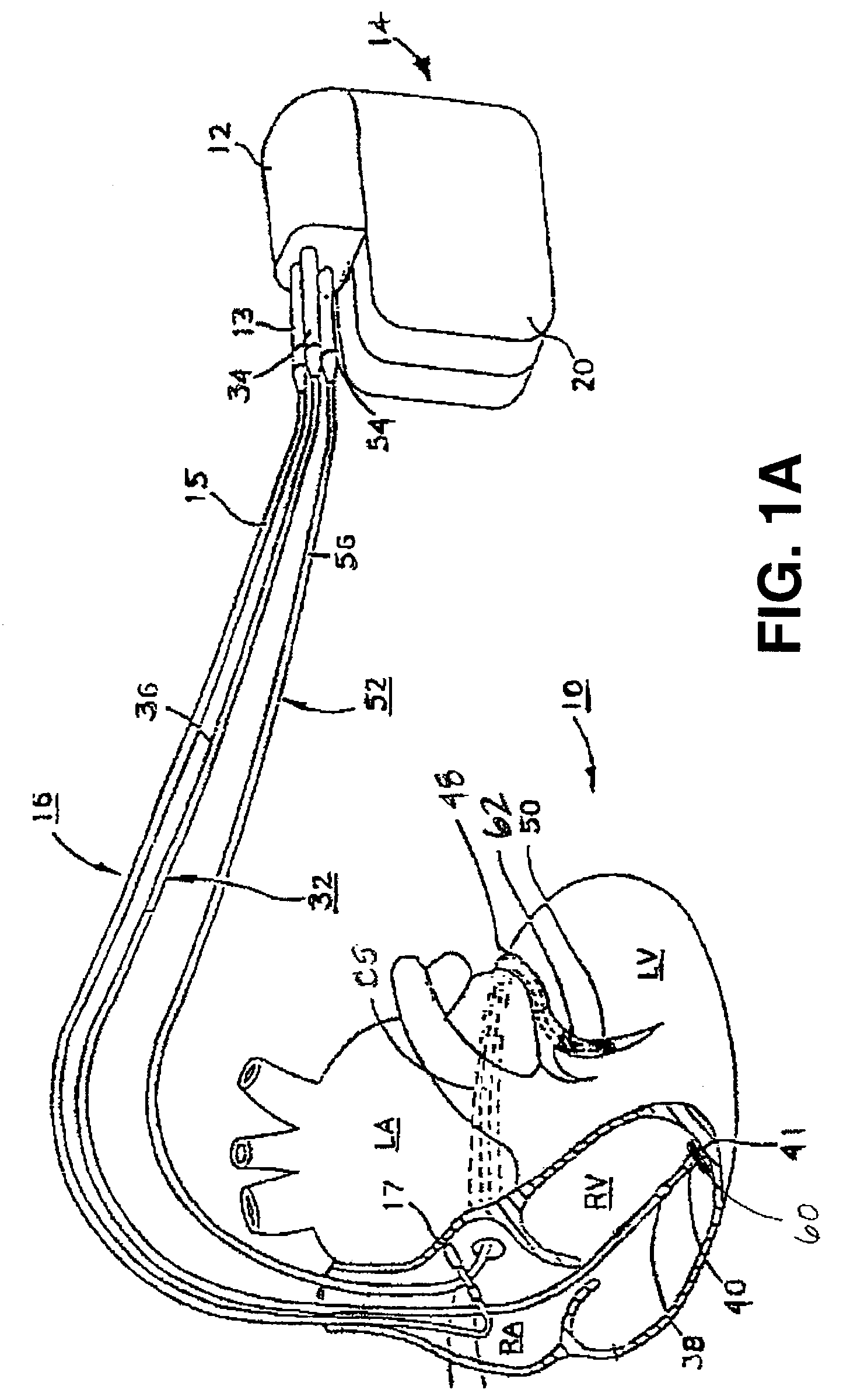
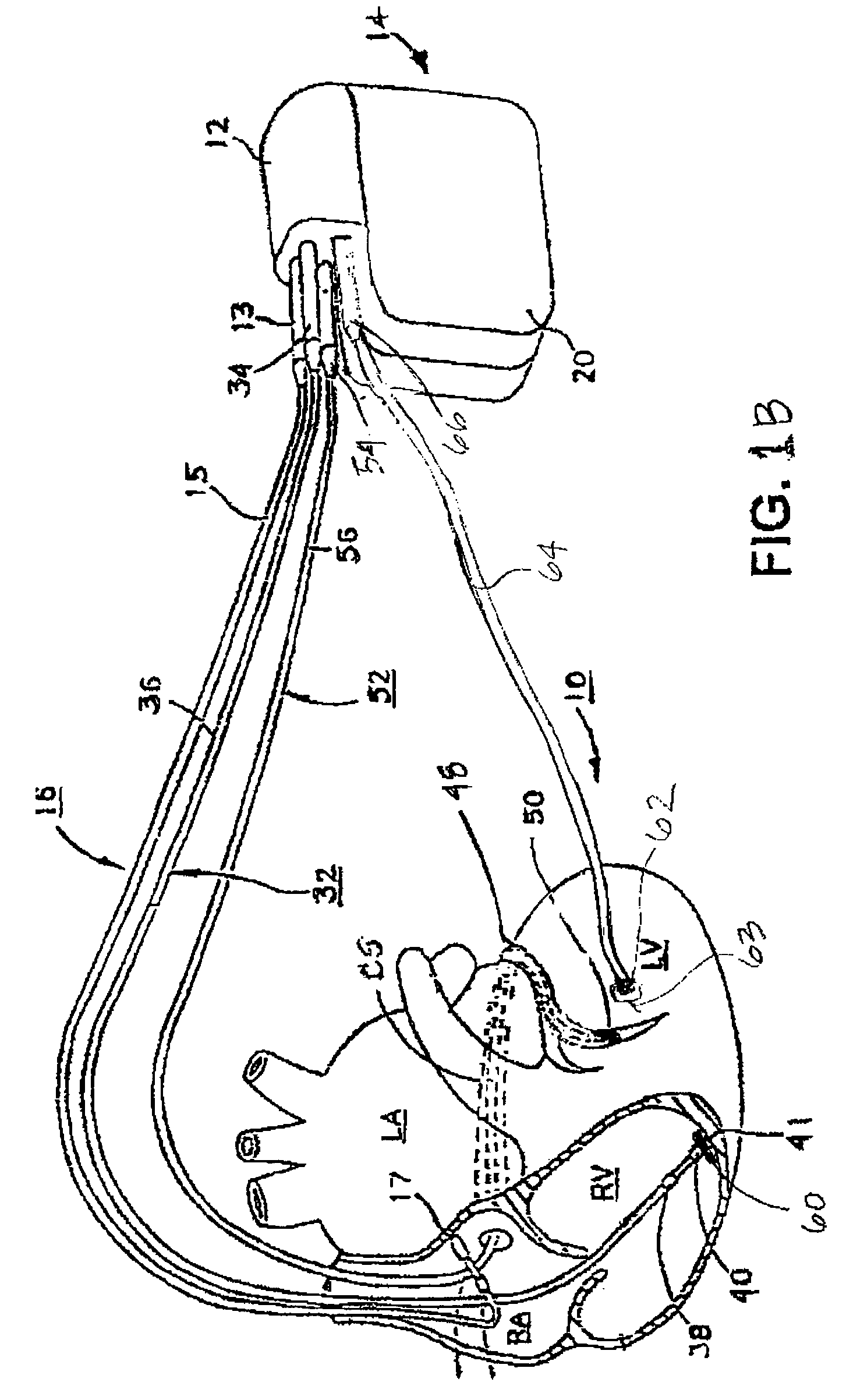
A system and method for monitoring left ventricular (LV) lateral wall motion and for optimizing cardiac pacing intervals based on left ventricular lateral wall motion is provided. The system includes an implantable or external cardiac stimulation device in association with a set of leads including a left ventricular epicardial or coronary sinus lead equipped with a motion sensor electromechanically coupled to the lateral wall of the left ventricle. The device receives and processes wall motion sensor signals to determine a signal characteristic indicative of systolic LV lateral wall motion or acceleration. An automatic pacing interval optimization method evaluates the LV lateral wall motion during varying pacing interval settings, including atrial-ventricular intervals and inter-ventricular intervals and selects the pacing interval setting(s) that correspond to LV lateral wall motion associated with improved cardiac synchrony and hemodynamic performance.
Owner:MEDTRONIC INC
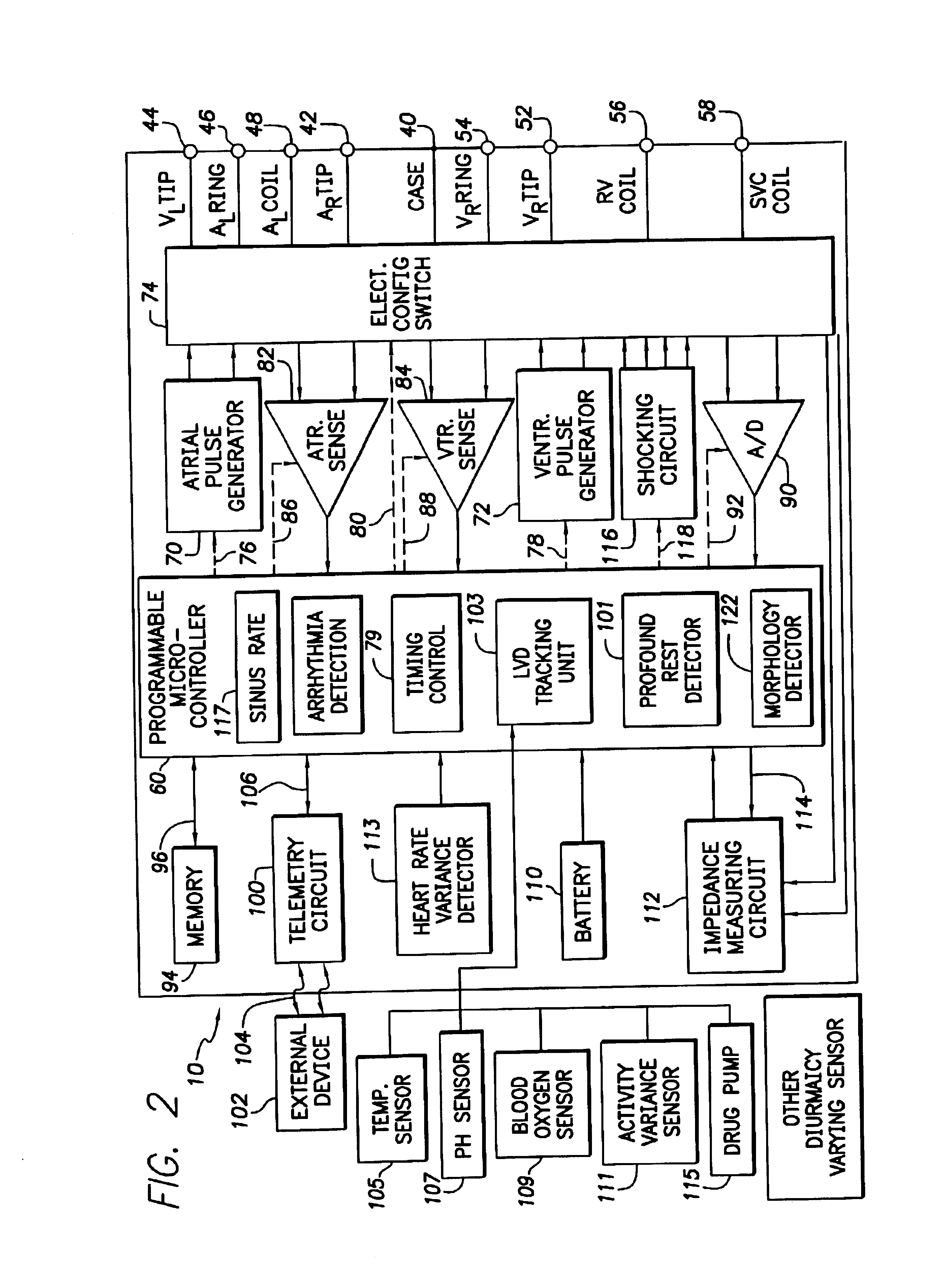
System and method for tracking progression of left ventricular dysfunction using implantable cardiac stimulation device
InactiveUS6922587B2Accurate and reliable assessmentAlter heart contractilityHeart stimulatorsPost extrasystolic potentiationCardiac pacemaker electrode
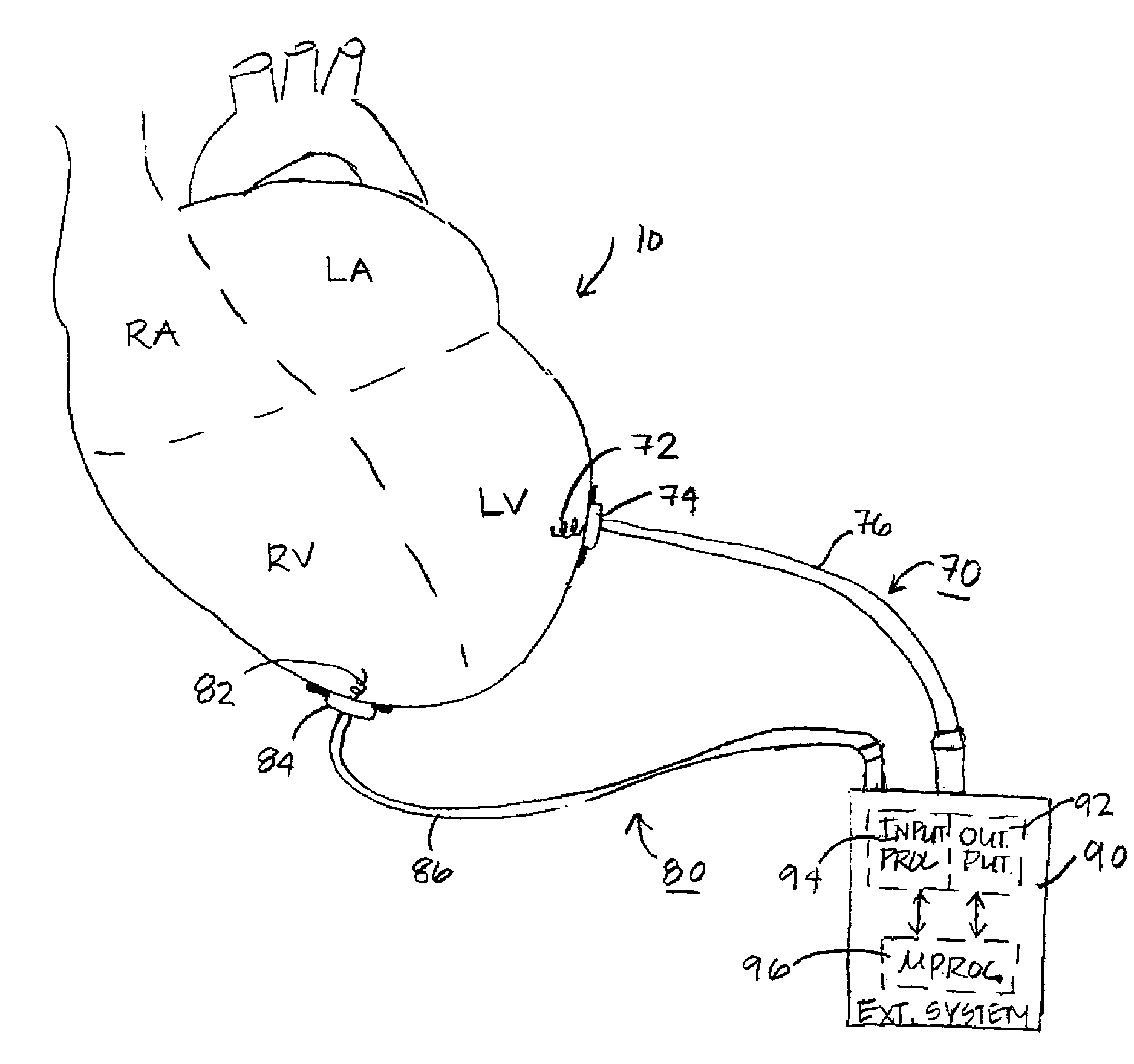
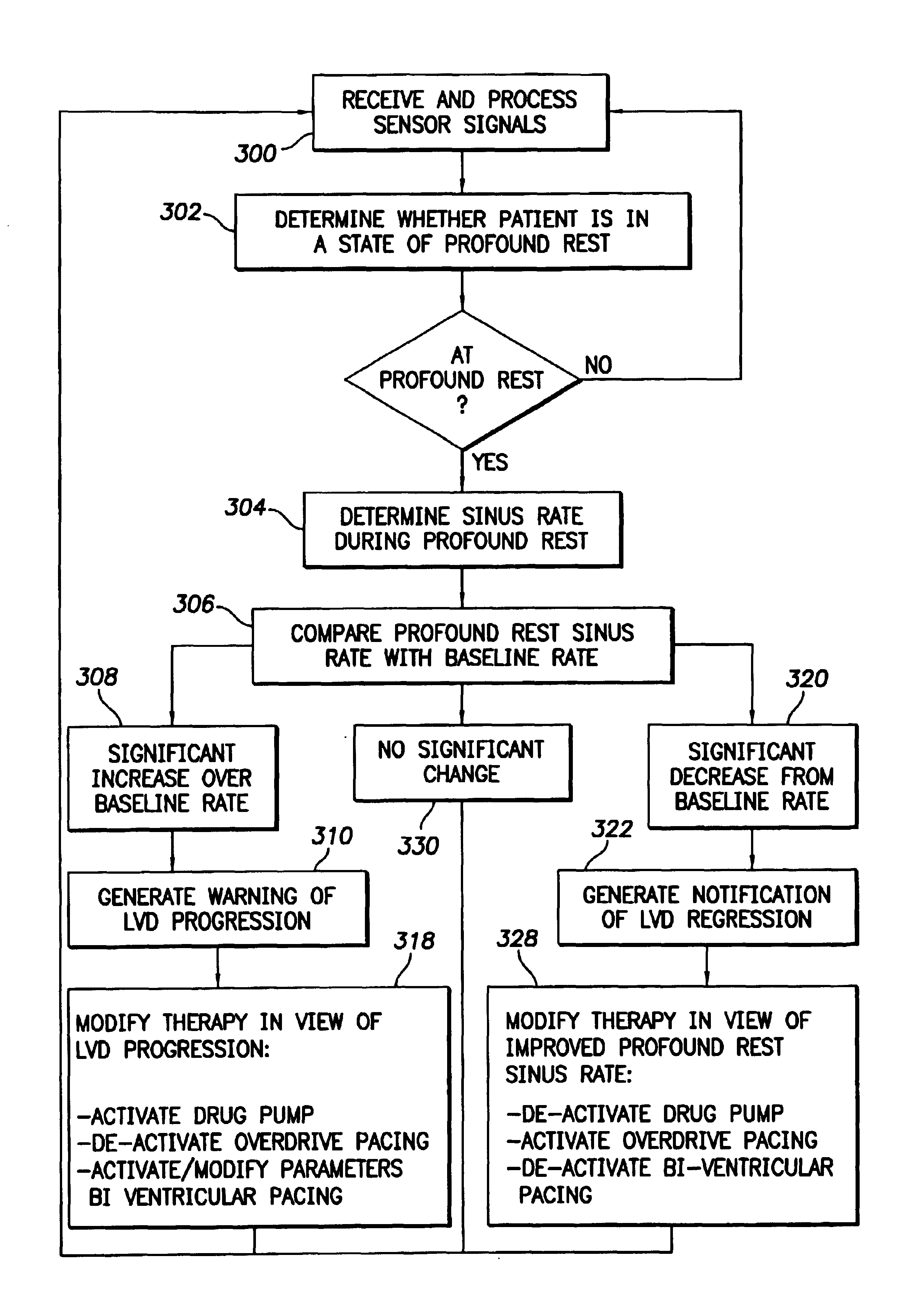
The progression or regression of left ventricular dysfunction (LVD) is automatically evaluated by a pacemaker or other implantable cardiac stimulation device by tracking changes in the resting sinus rate of the patient in which the device is implanted. The resting sinus rate is detected by first determining whether the patient is in a state of profound rest, such as sleep, then measuring the actual sinus rate during profound rest. Profound rest may be detected by using an activity variance sensor. An increase in the profound rest sinus rate over a period of several months indicates progression of LVD; whereas a decrease indicates regression. Appropriate LVD diagnostic information is recorded for subsequent review by a physician. Based on the progression or regression of LVD, the physician may then modify LVD drug therapy administered to the patient or may adjust control parameters of the pacemaker, such as overdrive pacing control parameters or control parameters affecting heart contractility via post-extrasystolic potentiation. If a drug pump is implanted within the patient for automatically delivering LVD drug therapy, the pacemaker controls the drug pump in view of any detected progression or regression of LVD. The technique may also be used to verify the efficacy of LVD drug therapy administered to the patient, whether delivered via an implanted drug pump or otherwise. Processing may be primarily performed within the implanted device itself or with an external programmer in communication with the implanted device. Activity state-based LVD tracking techniques are also set forth.
Owner:PACESETTER INC
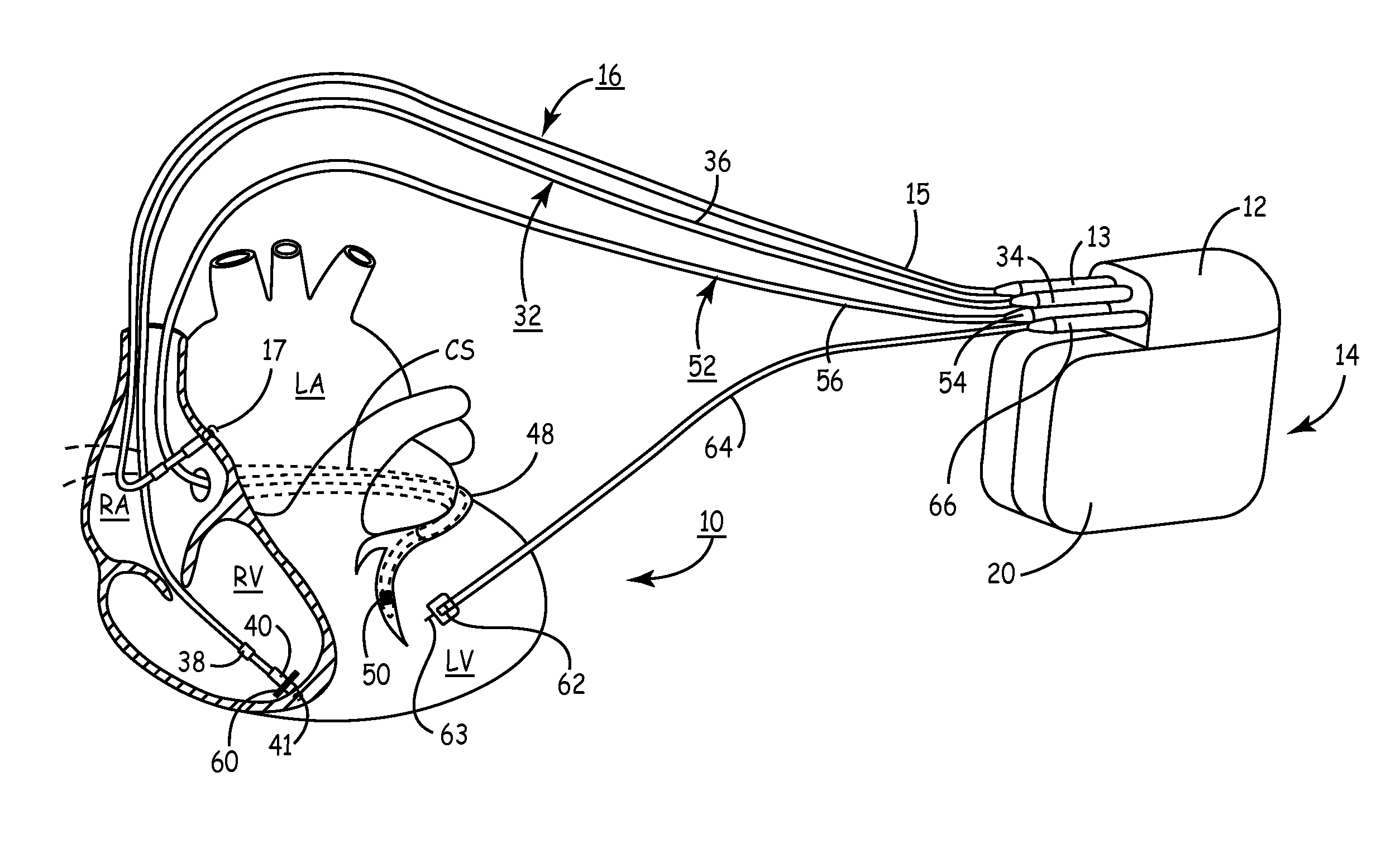
Method and apparatus for assessing left ventricular function and optimizing cardiac pacing intervals based on left ventricular wall motion
A system and method for monitoring left ventricular (LV) lateral wall motion and for optimizing cardiac pacing intervals based on left ventricular lateral wall motion is provided. The system includes an implantable or external cardiac stimulation device in association with a set of leads including a left ventricular epicardial or coronary sinus lead equipped with a motion sensor electromechanically coupled to the lateral wall of the left ventricle. The device receives and processes wall motion sensor signals to determine a signal characteristic indicative of systolic LV lateral wall motion or acceleration. An automatic pacing interval optimization method evaluates the LV lateral wall motion during varying pacing interval settings, including atrial-ventricular intervals and inter-ventricular intervals and selects the pacing interval setting(s) that correspond to LV lateral wall motion associated with improved cardiac synchrony and hemodynamic performance.
Owner:MEDTRONIC INC
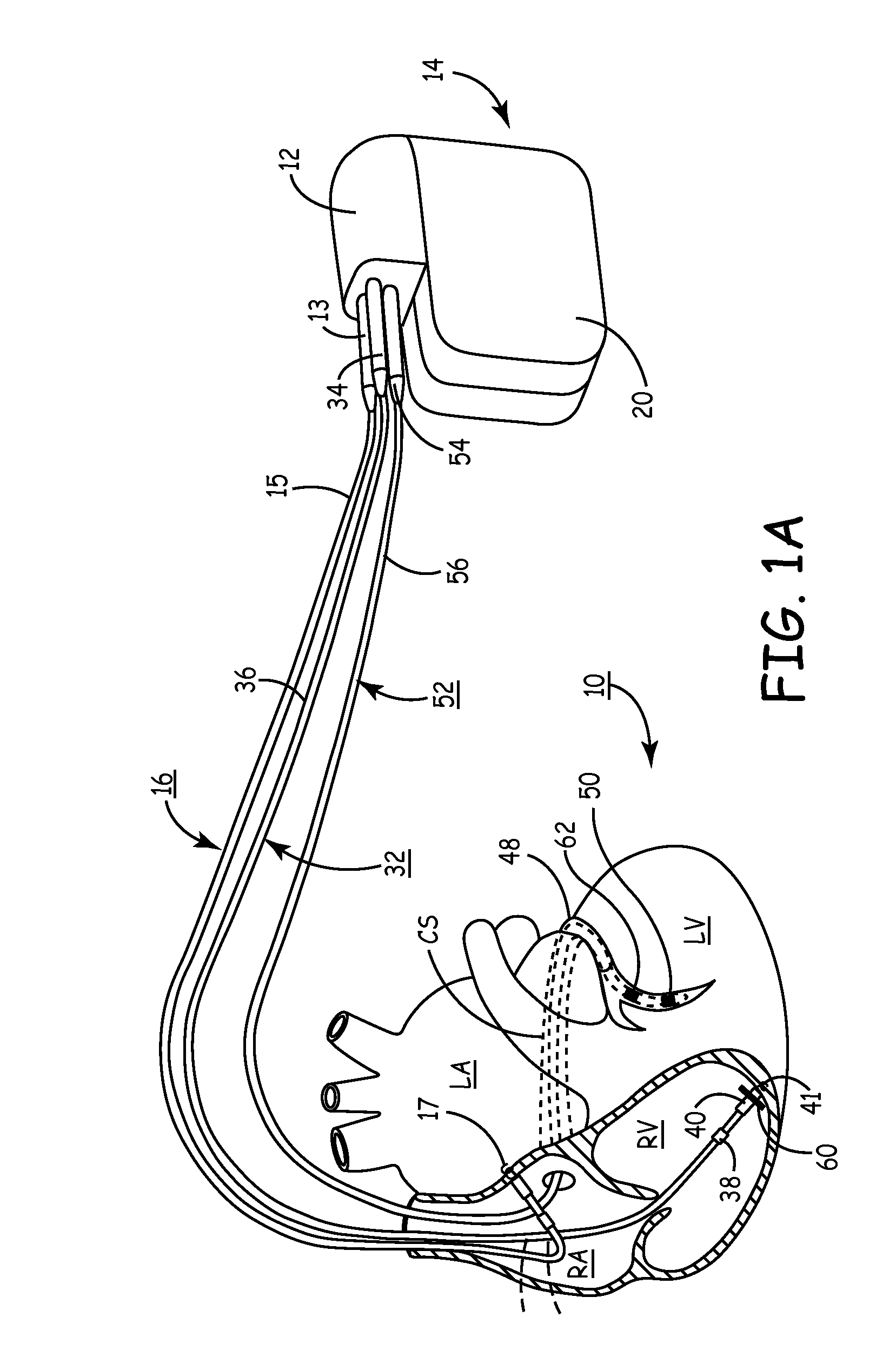
Method and apparatus for assessing left ventricular function and optimizing cardiac pacing intervals based on left ventricular wall motion
A system and method for monitoring left ventricular (LV) lateral wall motion and for optimizing cardiac pacing intervals based on left ventricular lateral wall motion is provided. The system includes an implantable or external cardiac stimulation device in association with a set of leads including a left ventricular epicardial or coronary sinus lead equipped with a motion sensor electromechanically coupled to the lateral wall of the left ventricle. The device receives and processes wall motion sensor signals to determine a signal characteristic indicative of systolic LV lateral wall motion or acceleration. An automatic pacing interval optimization method evaluates the LV lateral wall motion during varying pacing interval settings, including atrial-ventricular intervals and inter-ventricular intervals and selects the pacing interval setting(s) that correspond to LV lateral wall motion associated with improved cardiac synchrony and hemodynamic performance.
Owner:MEDTRONIC INC
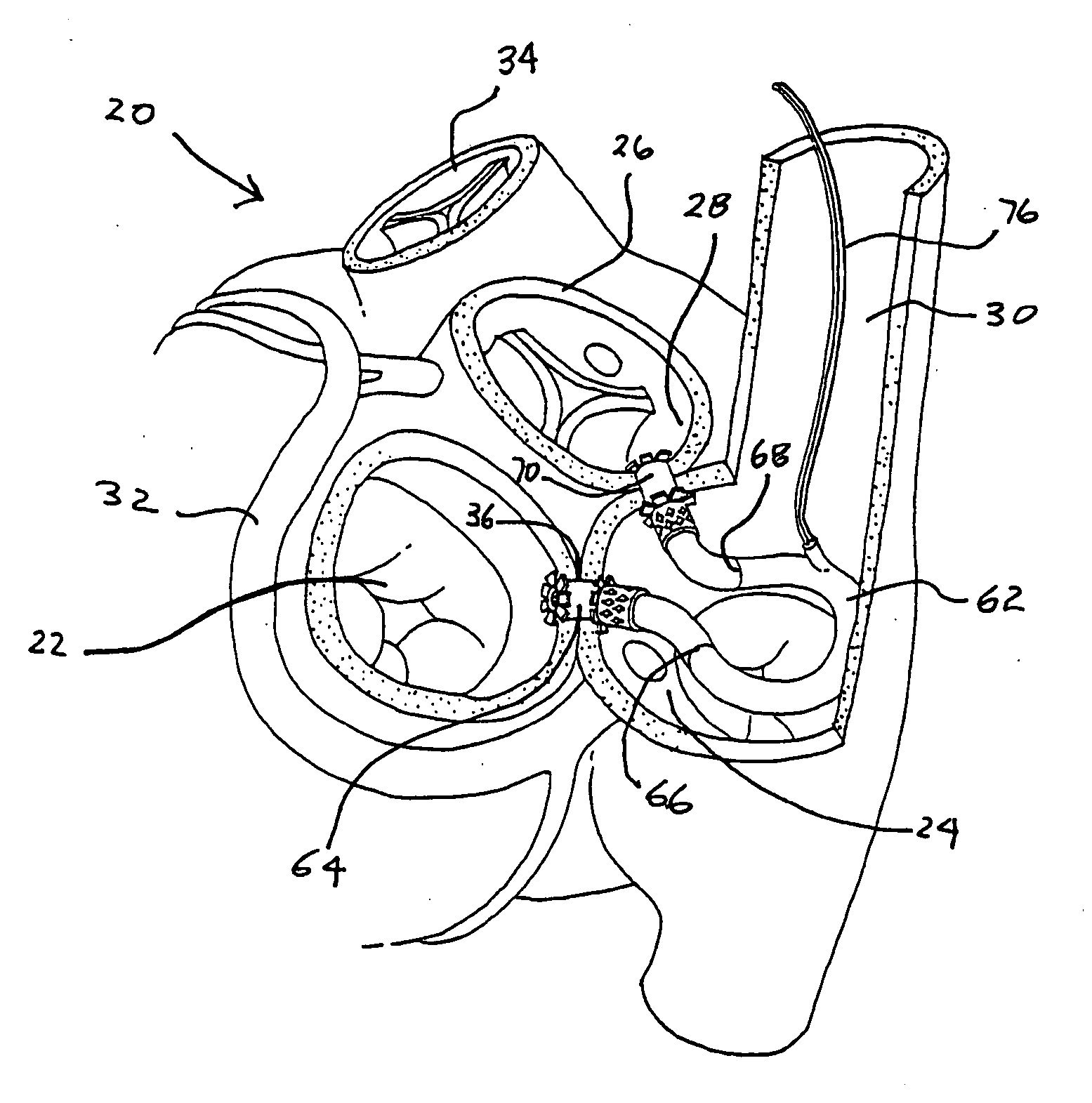
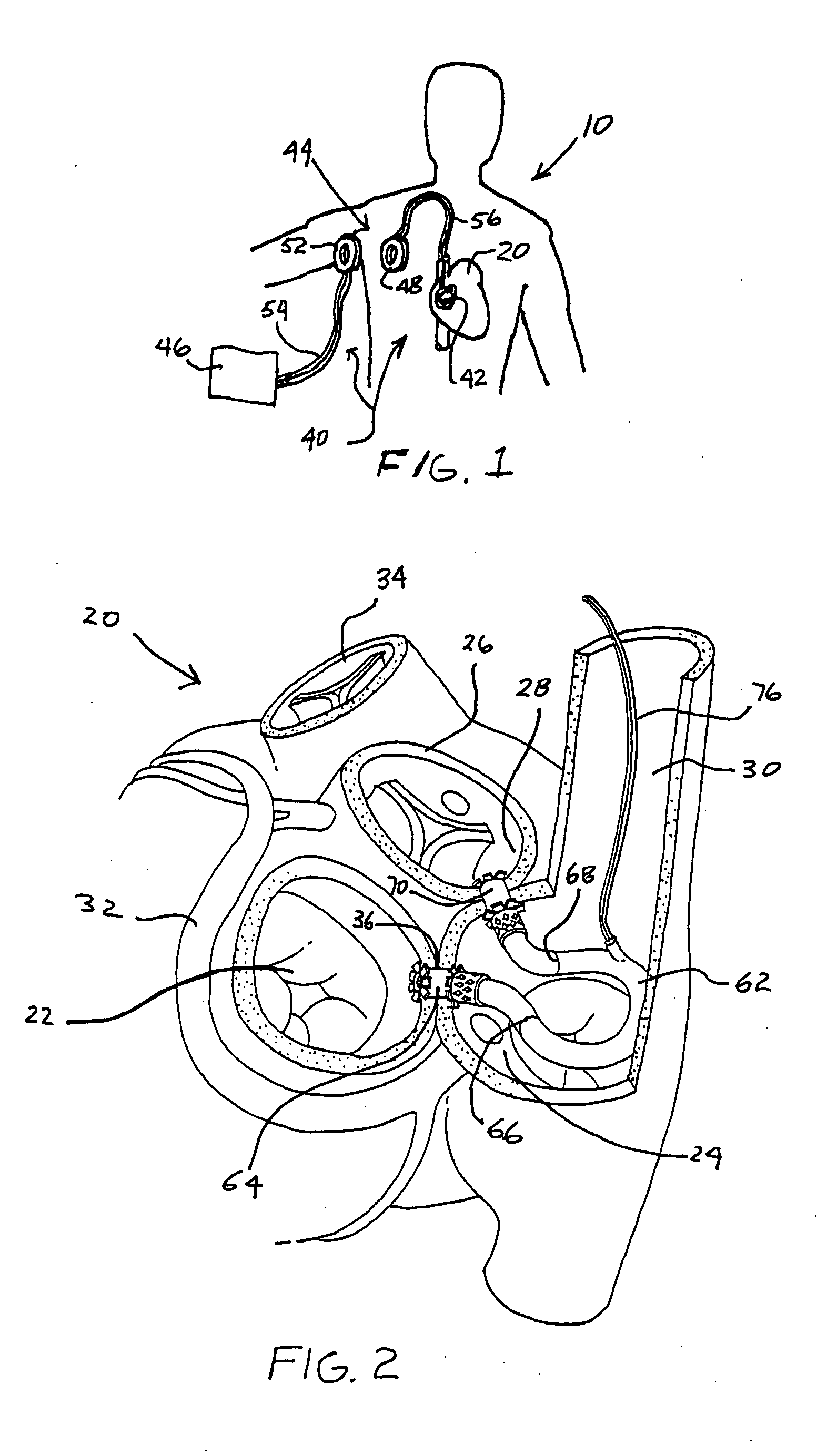
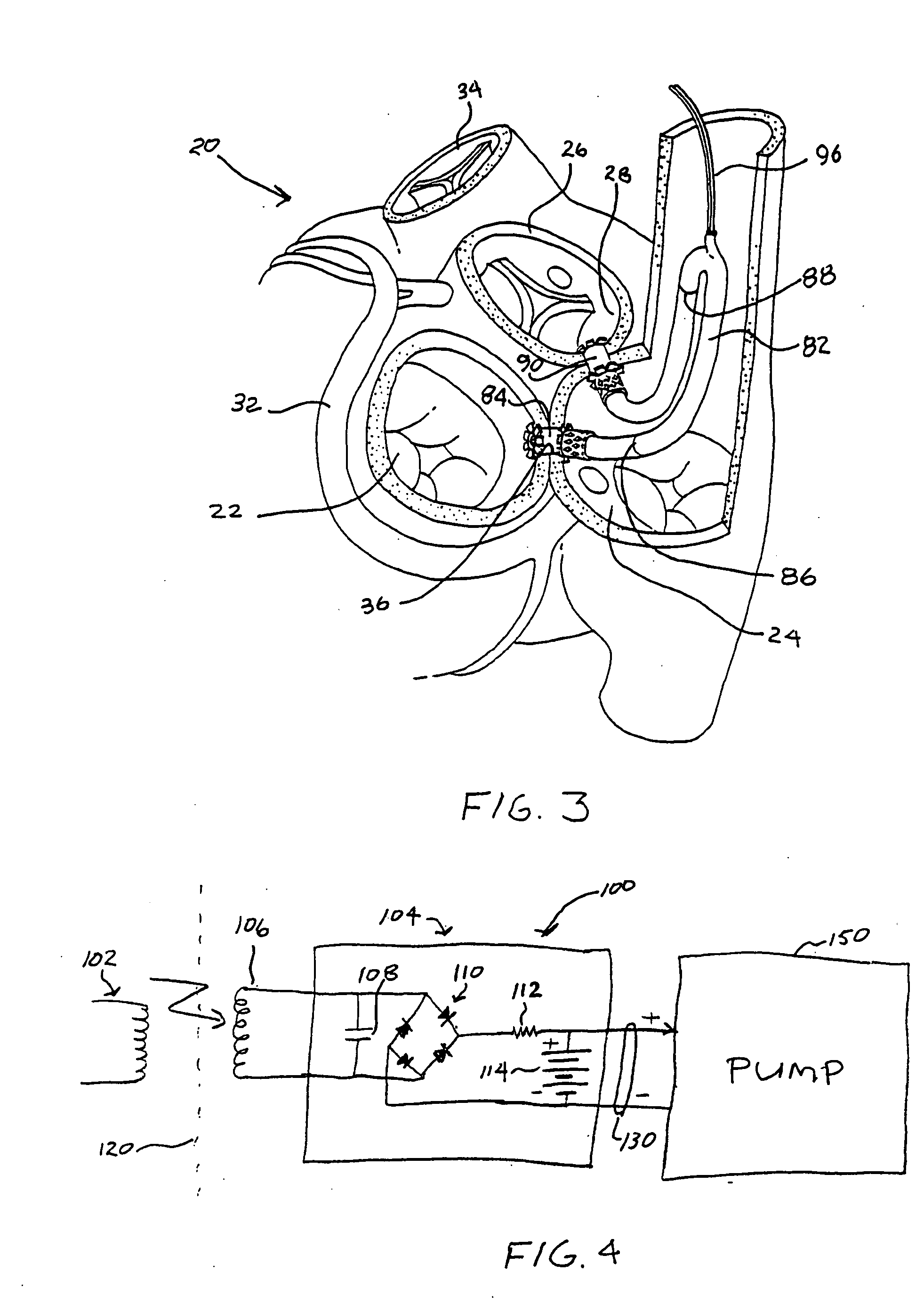
Left ventricular function assist system and method
InactiveUS20050187425A1Assist left ventricular functionBlood pumpsMedical devicesVeinLeft ventricular size
A pump assists left ventricular function of a heart. The pump is configured for implant in the body and has an input connectable to the left atrium and an output connectable to the aorta for pumping blood from the left atrium to the aorta. A power source for powering operation of the pump is connectable to the pump. The pump may be configured for implant within the heart, such as, for example, in the atrium, the super vena cava, or partly within each of the right atrium and the superior vena cava.
Owner:SCOUT MEDICAL TECH
Method for multiple site, right ventricular pacing with improved left ventricular function
InactiveUS20050203580A1Prevent and slow and reverse progressionImprove heart functionHeart stimulatorsVentricular outflow tractLeft ventricular size
A method for treatment of congestive heart failure from the right side of the heart, by stimulating at numerous points along the right-ventricular septum to produce a fused line of stimulation upon breakthrough of wave fronts into the left ventricular septum and an LV action potential that simultaneously propagates toward the apex, base, and left free wall. Five electrodes, all in contact with the septum and spaced approximately 1.5 cm apart, produce a fused action potential in an average adult human within 10 ms of delivering simultaneous pacing pulses. Breakthrough of this fused region of stimulation will occur within 20 ms of delivering the pacing pulses. The most proximal electrode may be located in or near the right-ventricular apex. The most distal electrode may be located somewhere near the right-ventricular outflow tract, generally somewhere near the moderator band.
Owner:QUETZAL BIOMEDICAL
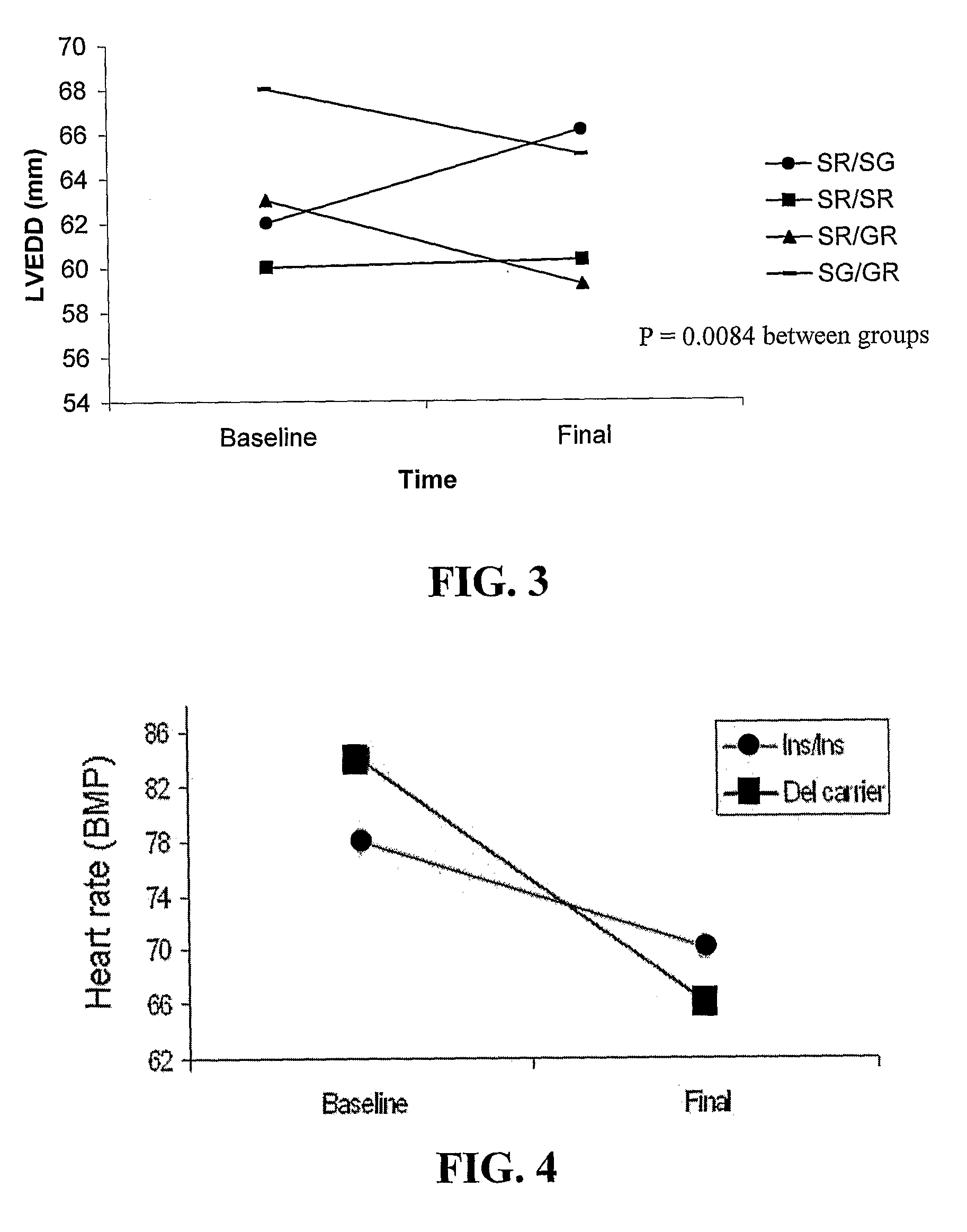
Device for and method of rapid noninvasive measurement of parameters of diastolic function of left ventricle and automated evaluation of the measured profile of left ventricular function at rest and with exercise
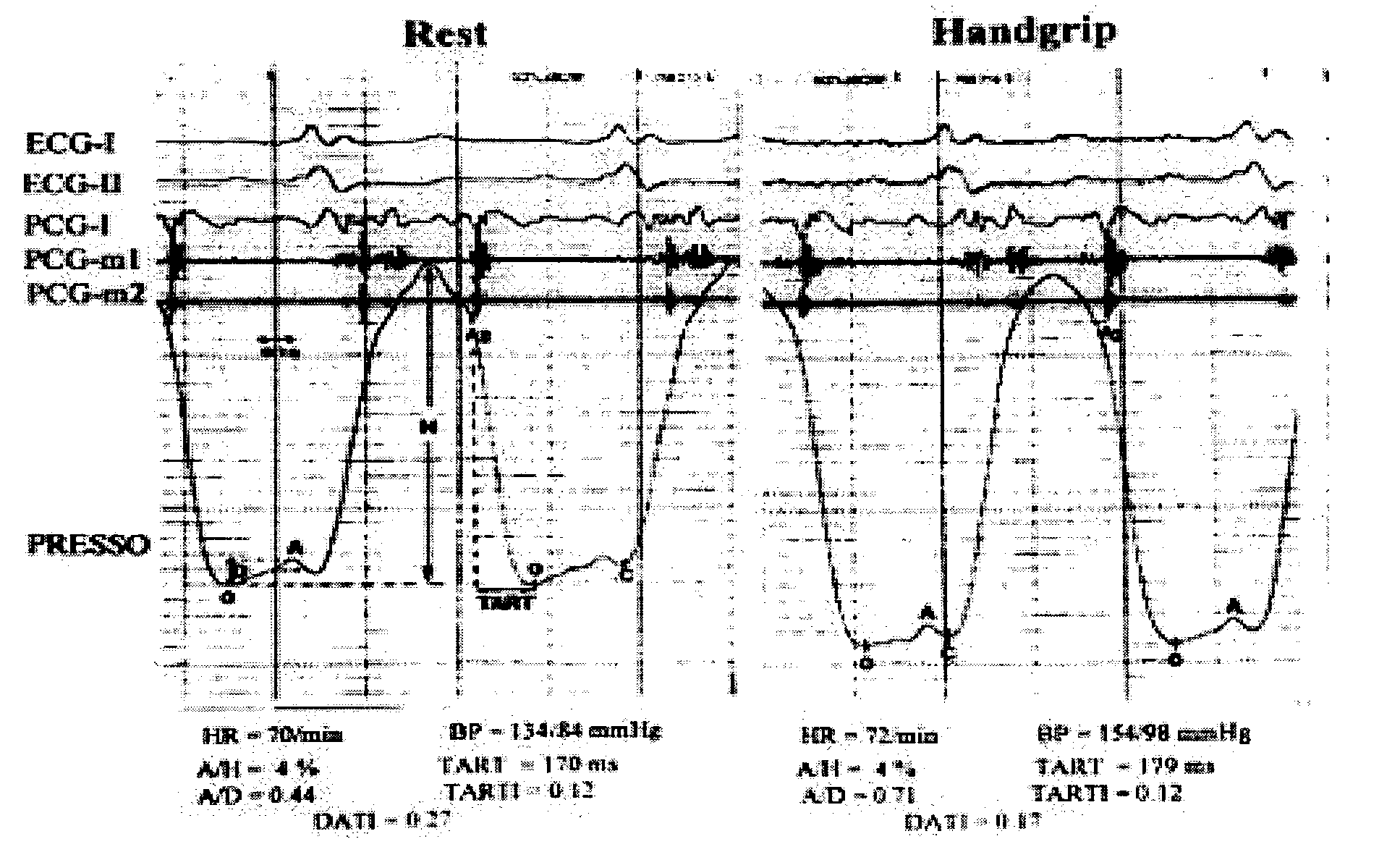
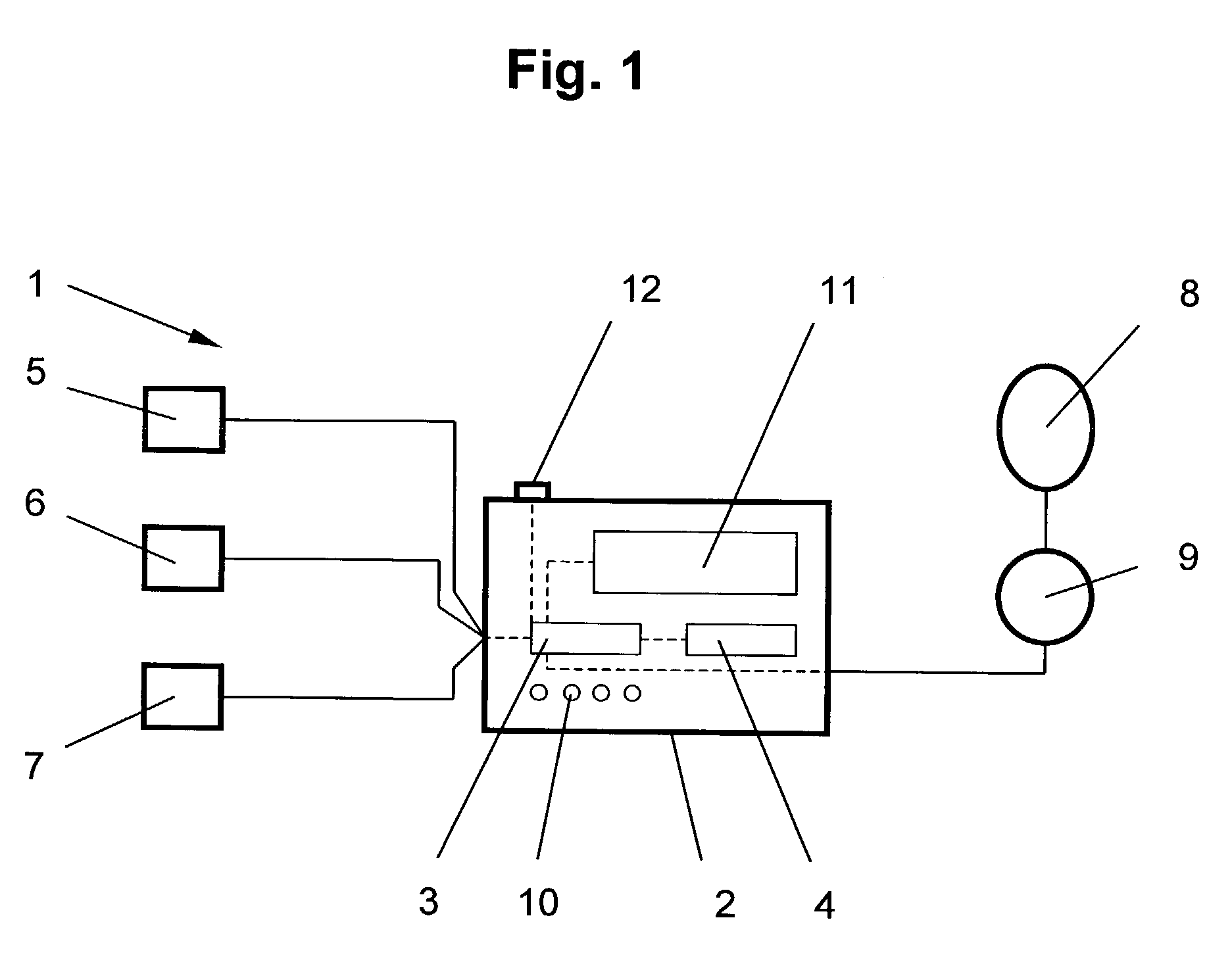
ActiveUS7107095B2Information can be usedSafe and convenient modalityEvaluation of blood vesselsAuscultation instrumentsLeft ventricular sizeHeart disease
In a method of noninvasive measurement of parameters of diastolic function of left ventricle and automated evaluation of the measured profile at rest and with exercise, a patient performs an isometric exercise. An external pressure sensor and heart sounds microphone are applied in a non-invasive manner on the thoracic wall to obtain a left ventricular pressure mirroring curve (pressocardiogram) and simultaneously the heart sounds (phonocardiogram). An external unit determines and calculates characteristic diastolic parameters derived from the pressocardiographic curve and phonocardiogram at rest, during and after exercise, converts each said pressocardiogram into a digital waveform in the time domain, and automatically categorizes the mentioned characteristic parameters based on exact categorization criteria for defining several differentialforms of diastolic dysfunction of left ventricle in human beings.
Owner:MANOLAS JAN
External counter pulsation treatment
A method for treating patients suffering from left ventricular dysfunction is disclosed. The method involves applying, during diastole, for a time period of about one hour, about five days each week for at least about seven weeks, an incrementally increasing therapeutic pressure to the patients' lower extremities, from the calves through the thighs and the buttocks. The initial hourly treatment is carried out at a peak diastolic / systolic pressure ratio (D / S Ratio) in the range of about 0.4:1 up to about 0.6:1 and thereafter at a D / S Ratio in the range of 0.5:1 to 1:1 for each consecutive hourly treatment, with the proviso that the average D / S Ratio over the entire treatment regimen does not exceed about 0.9:1.
Owner:CARDIOMAX
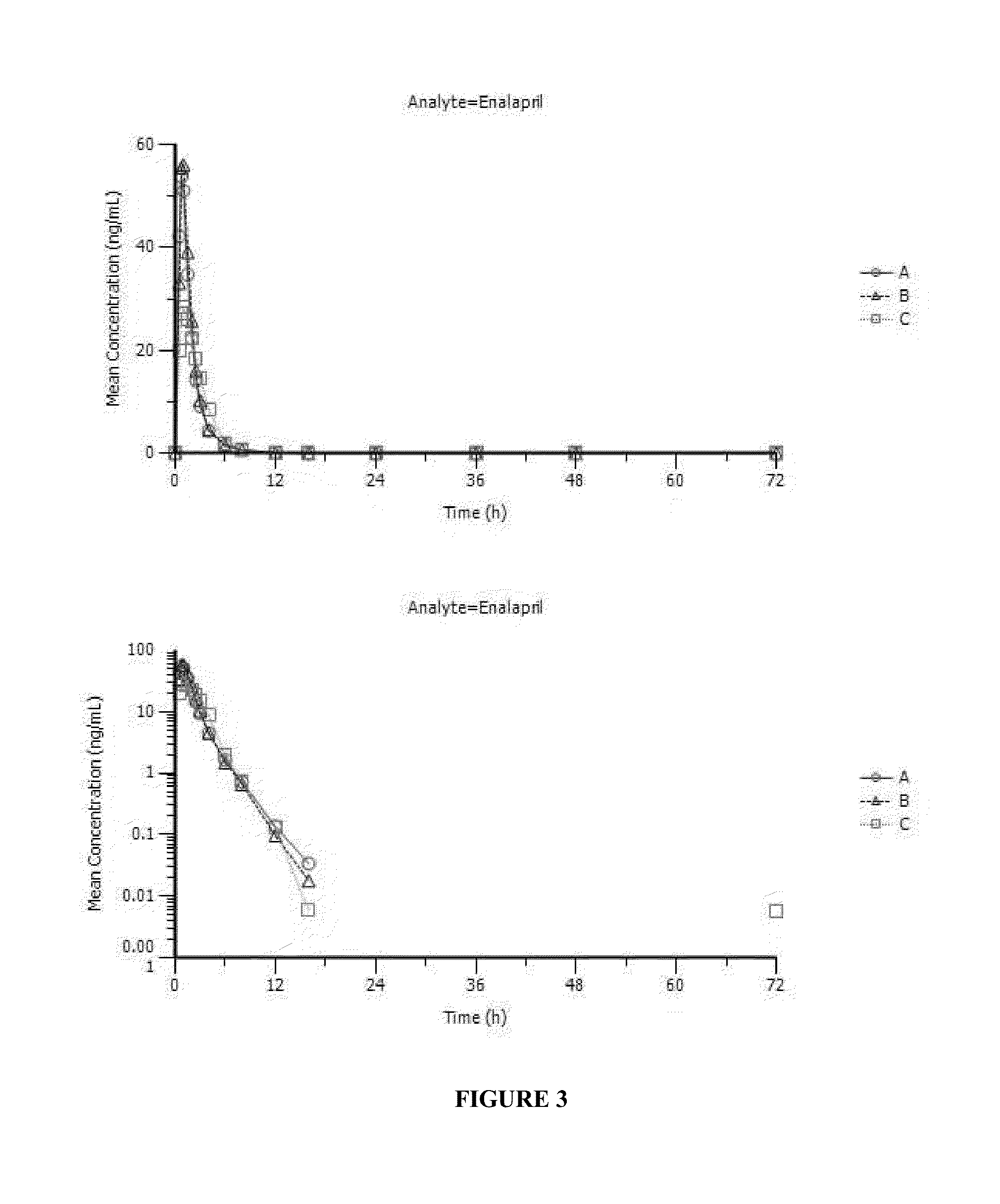
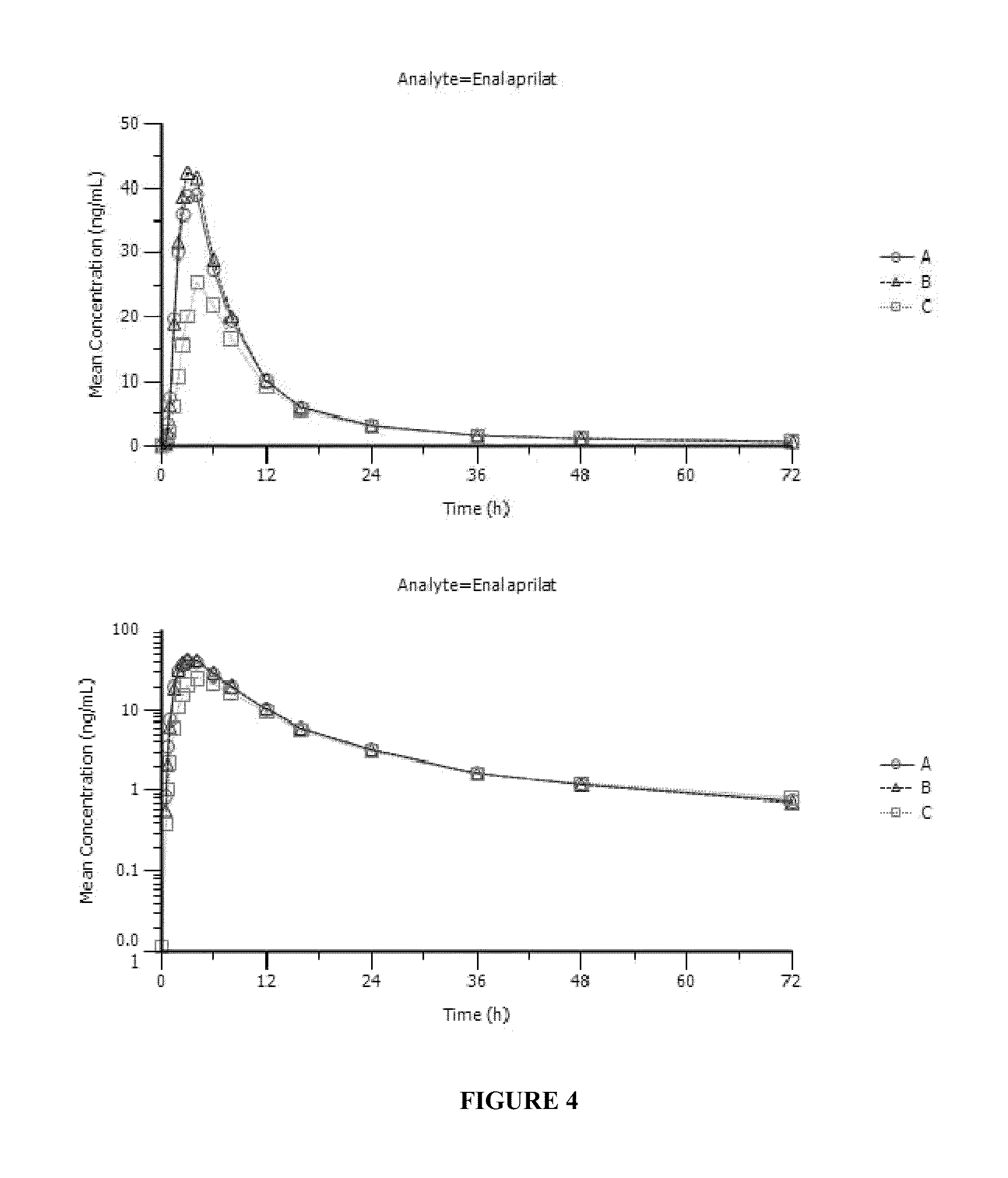
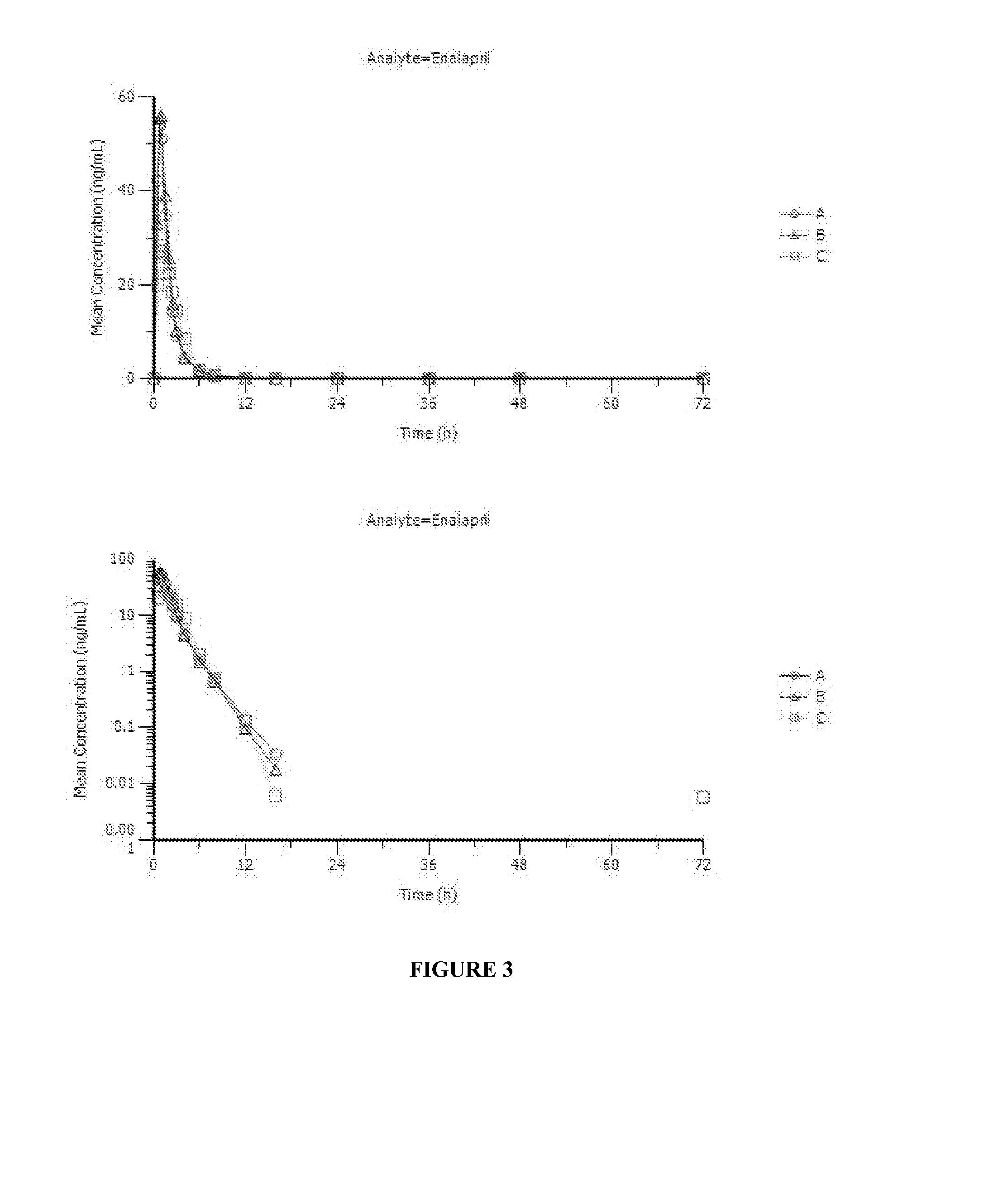
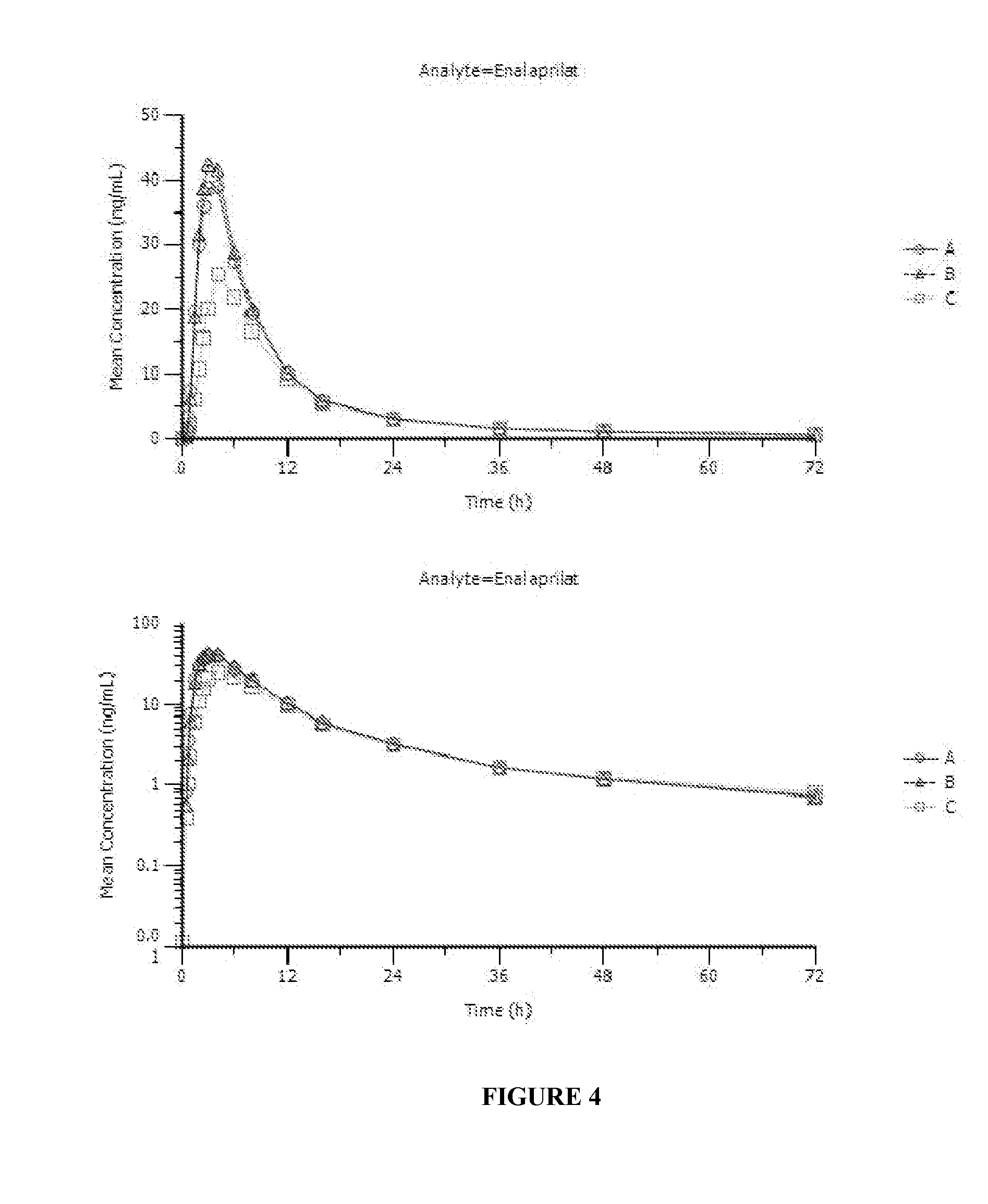
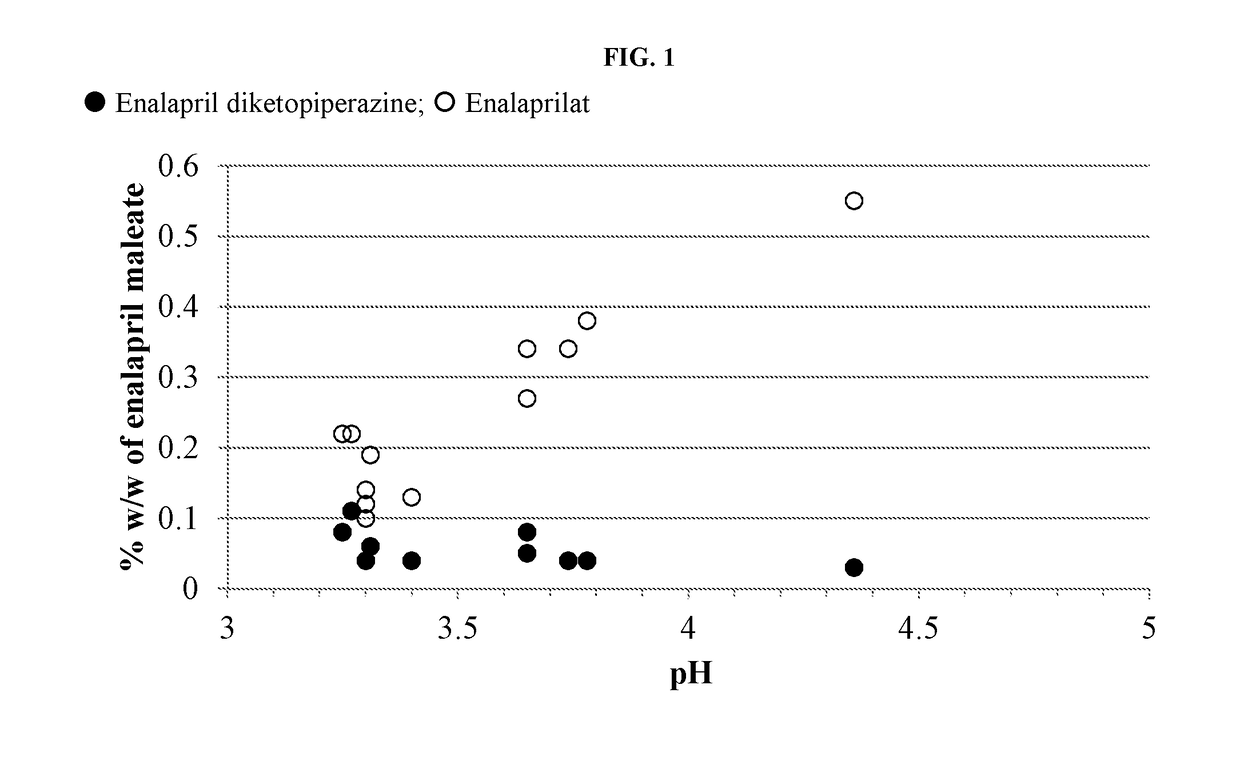
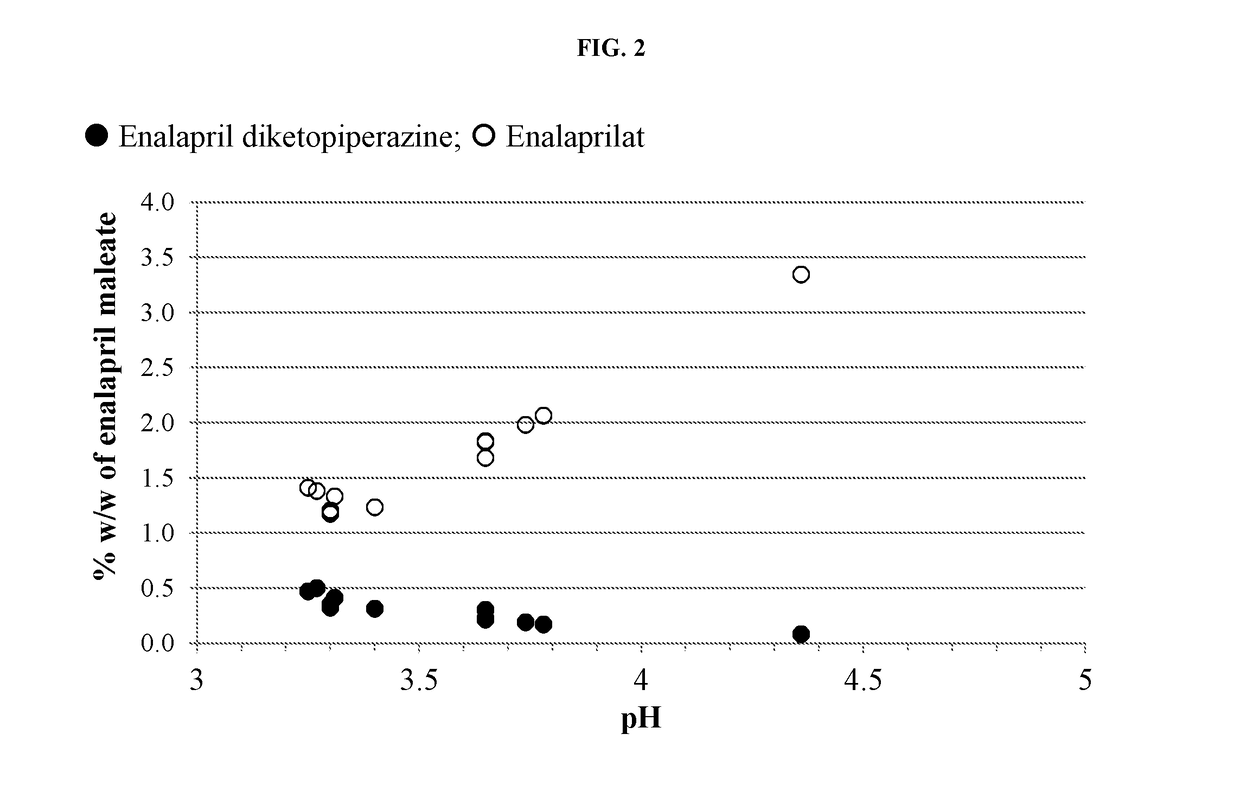
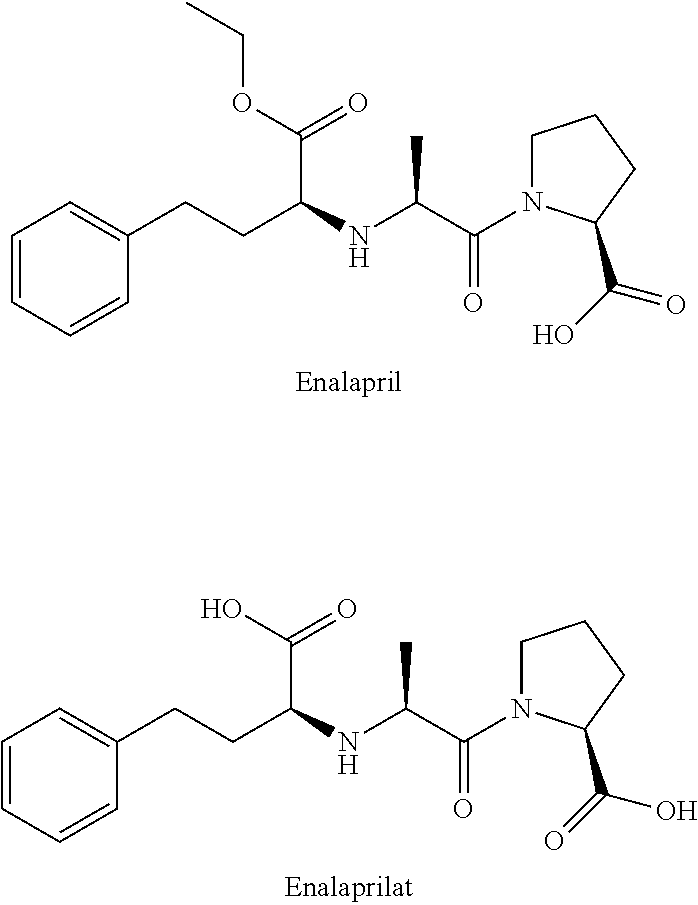
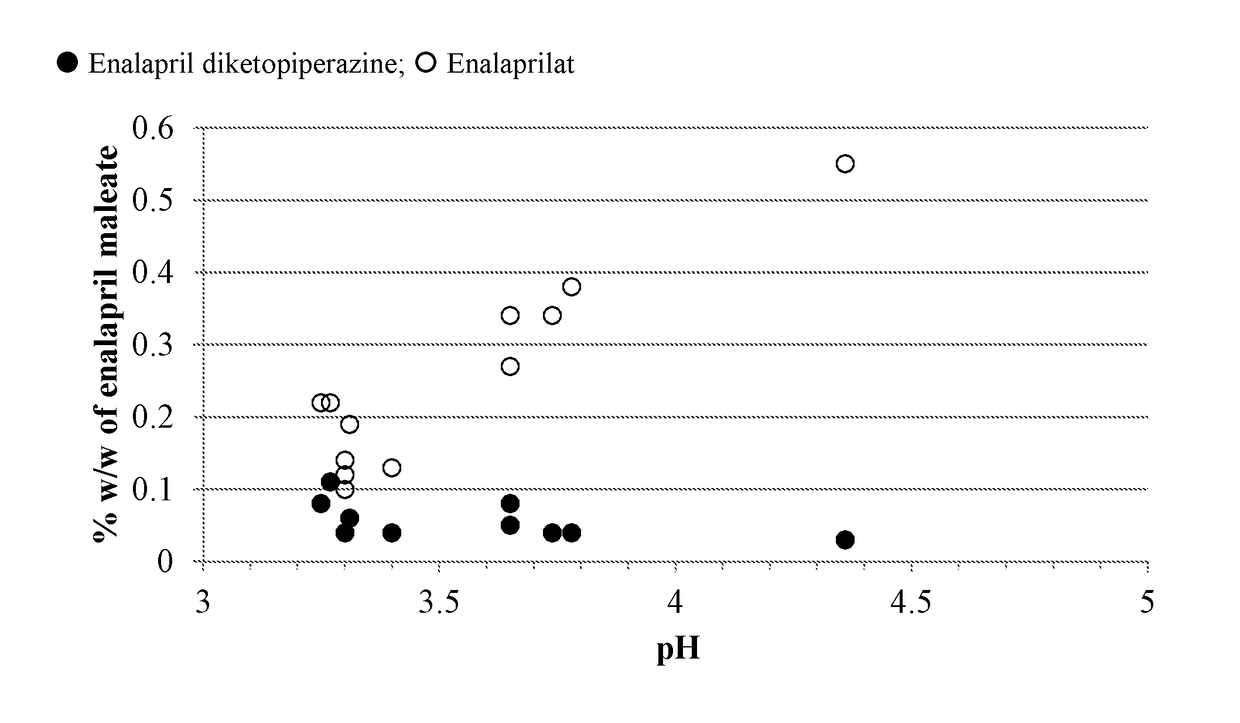
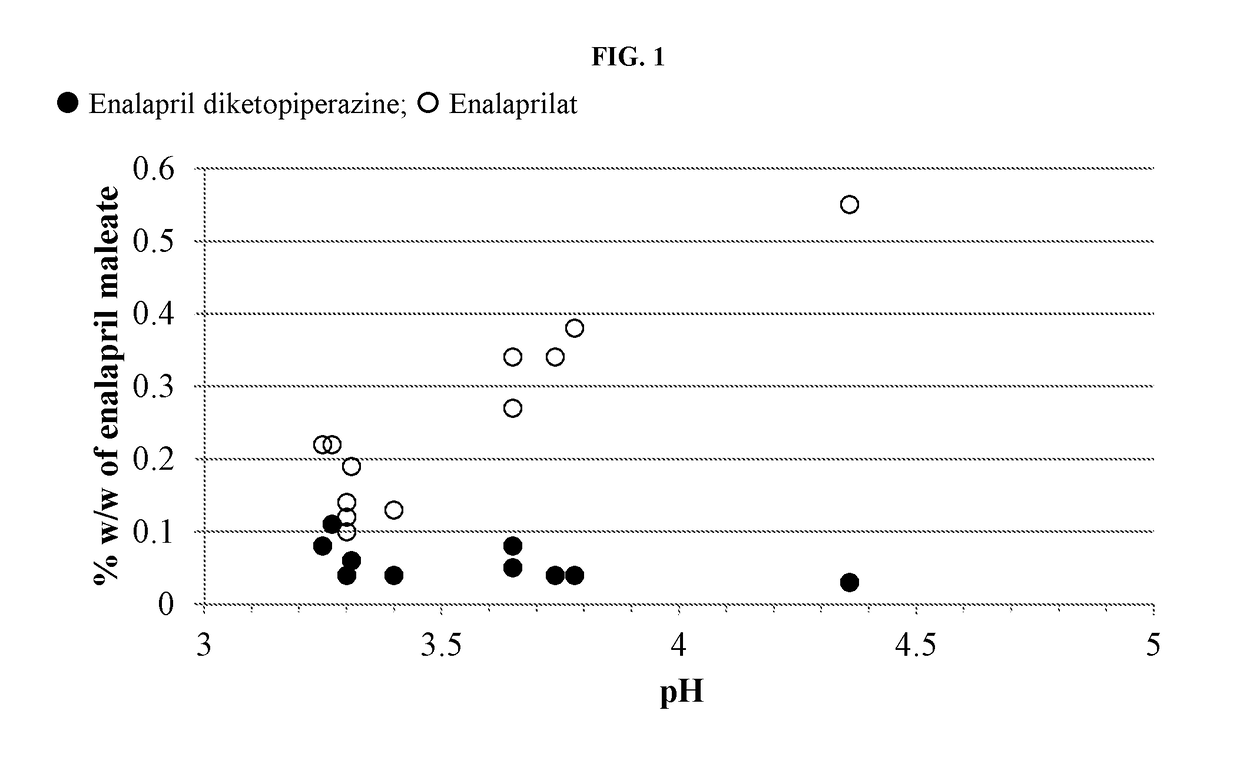
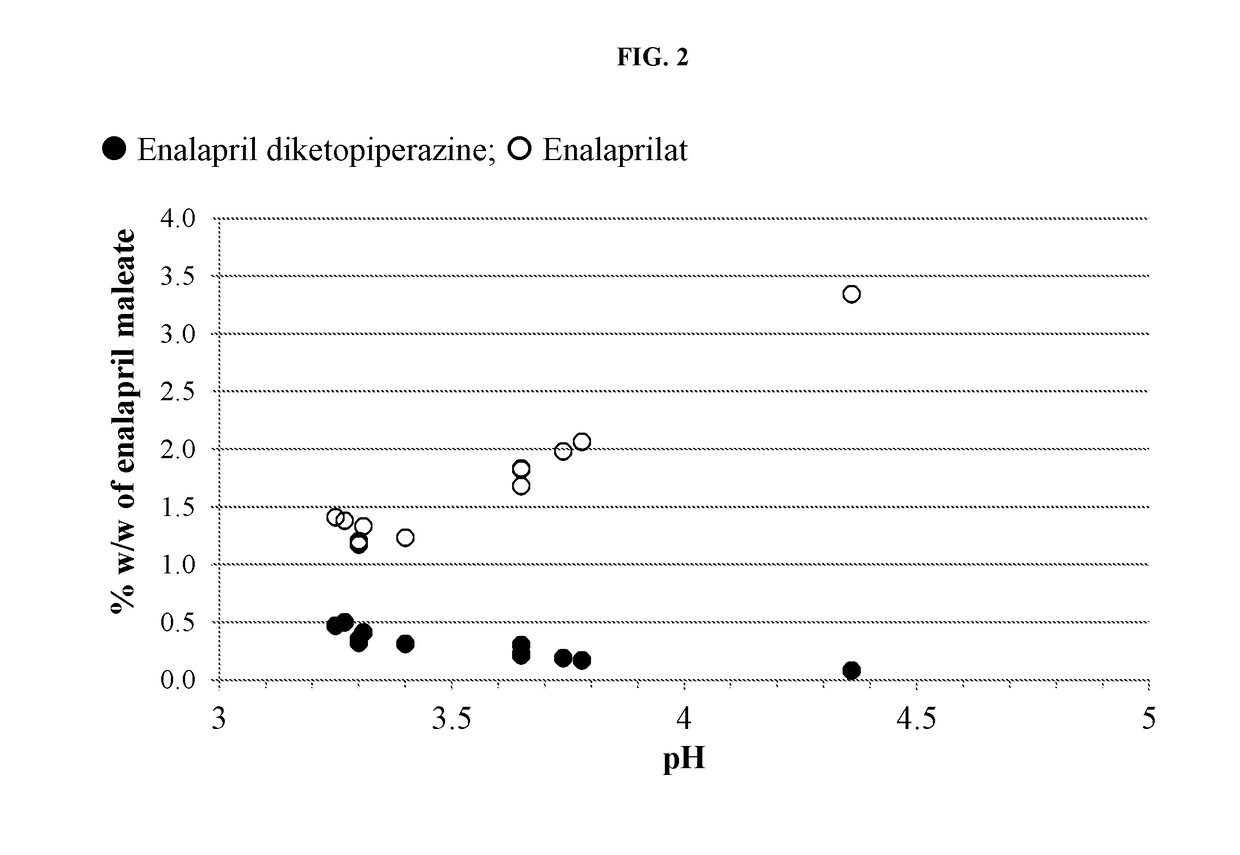
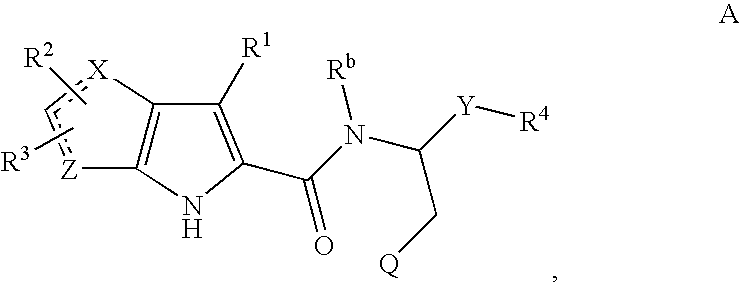
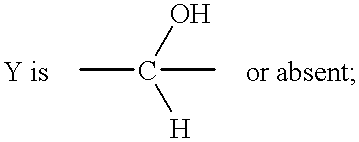
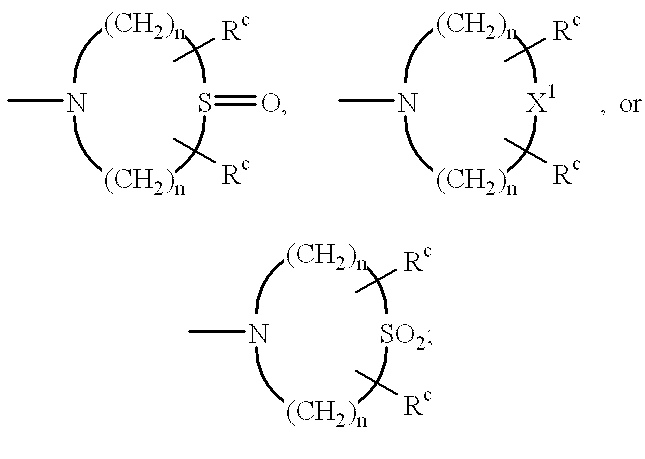
Enalapril compositions
Provided herein are stable enalapril powder compositions for oral liquid formulation. Also provided herein are methods of using enalapril oral liquid formulations for the treatment of certain diseases including hypertension, heart failure and asymptomatic left ventricular dysfunction.
Owner:UNIVERSITY OF KANSAS +1
Enalapril Compositions
Owner:UNIVERSITY OF KANSAS +1
External counter pulsation treatment
A method for treating patients suffering from left ventricular dysfunction, exhibited by a left ventricular ejection fraction (LVEF) less than normal, is disclosed. The method involves applying, during diastole, for a time period of about one hour, about five days each week for at least about seven weeks, an incrementally increasing therapeutic pressure to the patients' lower extremities, from the calves through the thighs and the buttocks. The hourly treatments are carried out at incrementally increasing peak diastolic / systolic pressure ratios (D / S Ratios) in the range of about 0.4:1 up to about 0.6:1 and thereafter at a D / S Ratio in the range of 0.5:1 to 1:1 for each consecutive hourly treatment, with the proviso that the average D / S Ratio over the entire treatment regimen does not exceed about 0.9:1.
Owner:CARDIOMAX
Removable left ventricular assist device with an aortic support apparatus
InactiveUS6228018B1Prevent the collapse of the walls of the aortaProlonged ejectionStentsControl devicesSystoleThumb opposition
An apparatus and method of temporarily replacing the function of the left ventricle in a patient whose heart is severely injured and is unable to maintain a systemic arterial pressure adequate to support the inside walls of patient's aorta, the method comprising a removable pressurizable support means having an external profile which is expandable to fit firmly against the inside wall of the aorta of a patient, wherein the external profile of the pressurizable support means presents a central opening that allows blood to flow through the aorta. The pressurizable support means can both support and expand the aorta under low blood pressure to assist the drawing of blood from the left ventricle. The central opening also allows for the placement of a blood flow control means. The blood flow control means can comprise a pumping balloon and a proximal blocking balloon, the two members pressurized and depressurized in opposition within the aorta of a patient to simulate systole and diastole of a healthy heart. The pumping balloon has a pressurized pumping position that is engaged while the proximal blocking balloon is in a depressurized deflated position.
Owner:NORTH TEXAS HEALTH SCIENCE CENTER AT FORT WORTH THE UNIVERSITY OF
Methods, Compositions, and Formulations for Preventing or Reducing Adverse Effects in a Patient
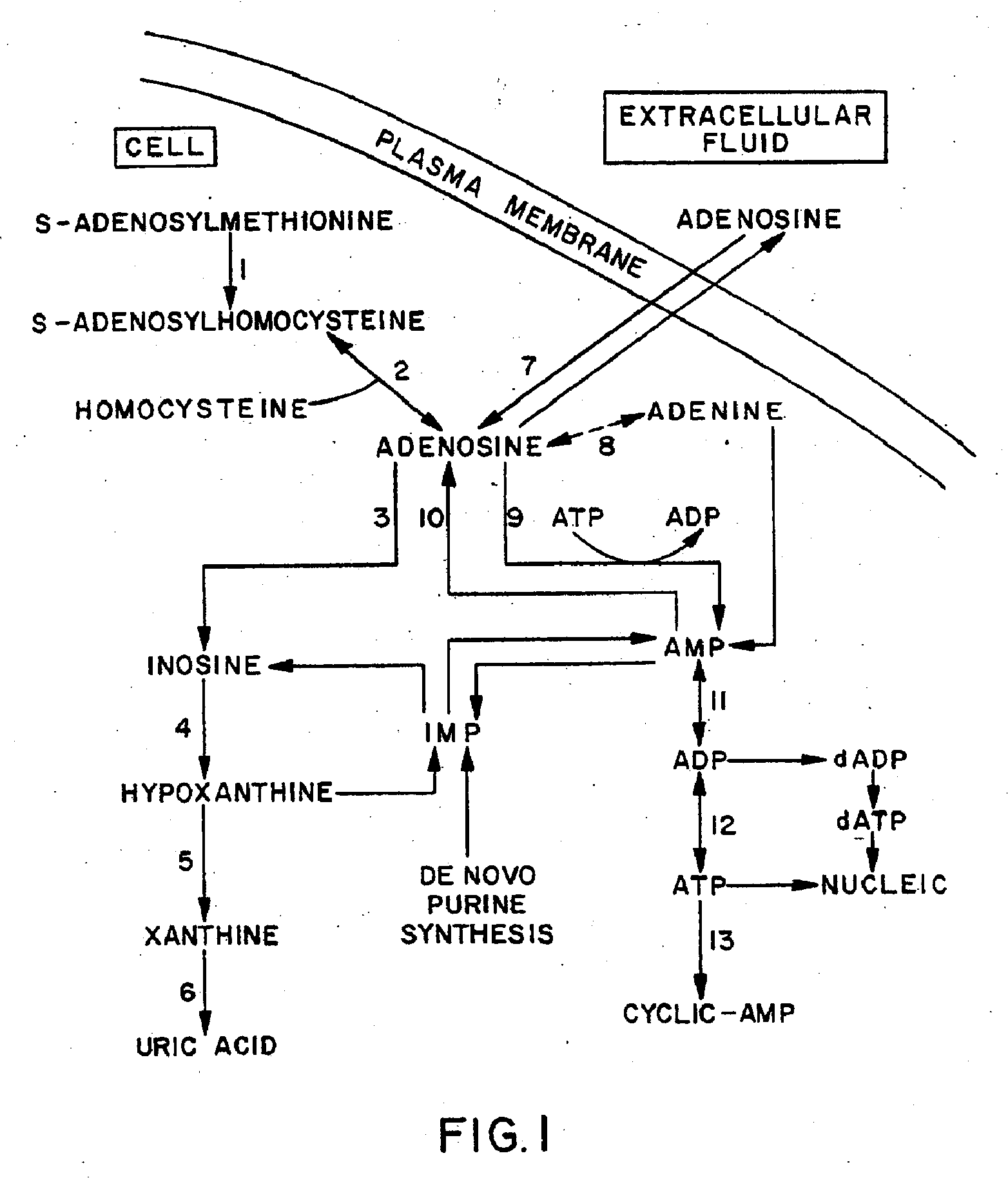
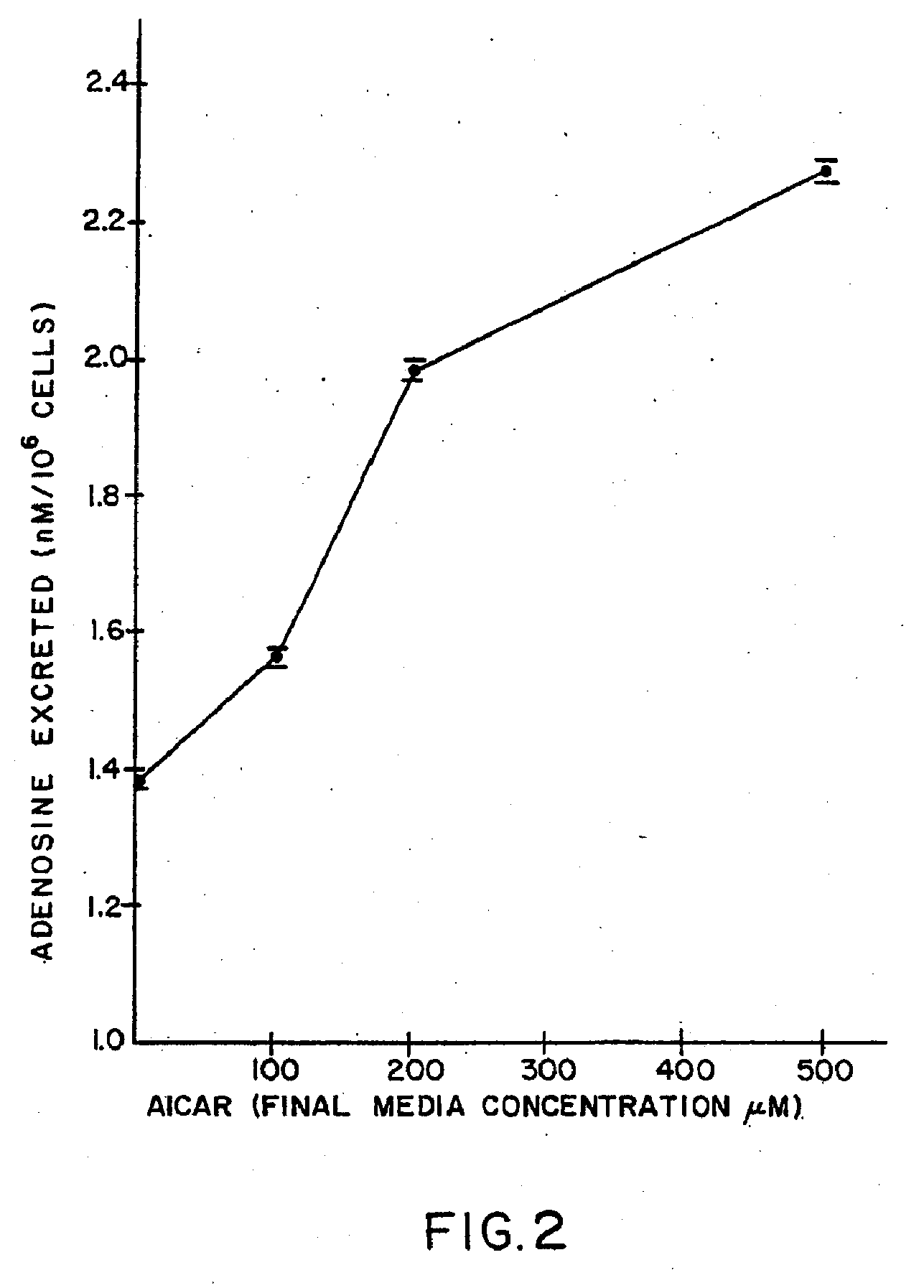
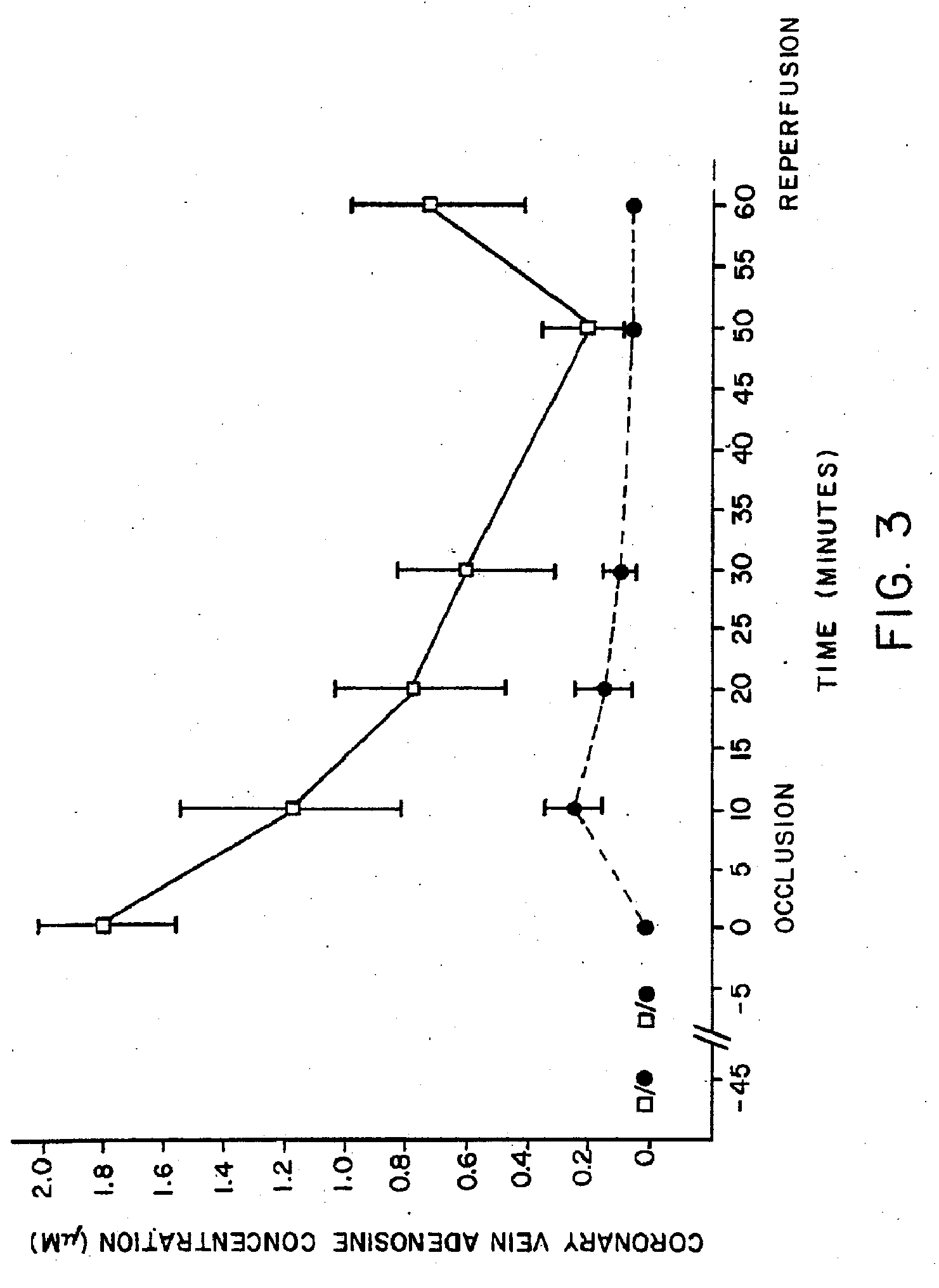
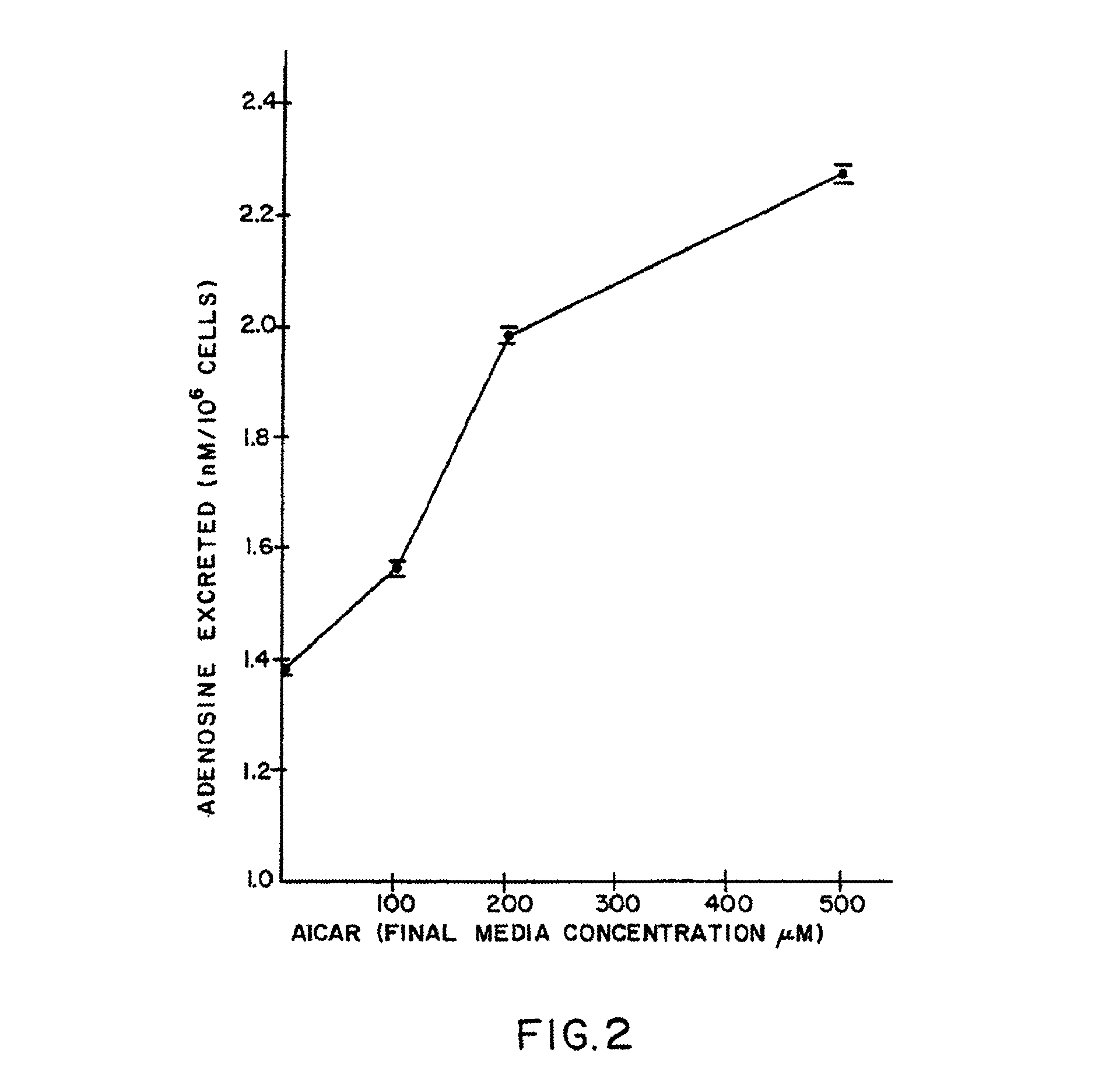
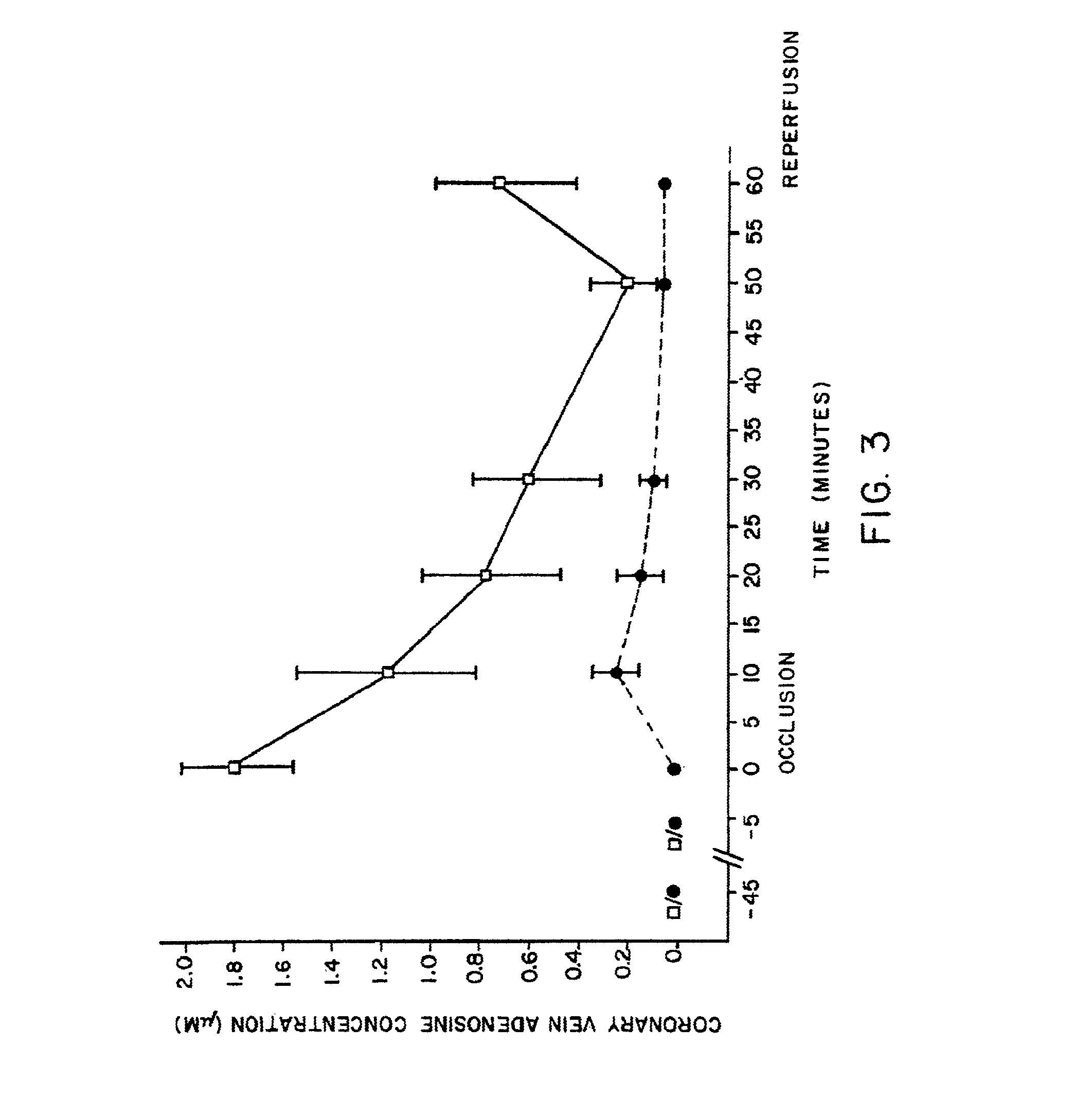
The present invention provides methods, compositions, formulations, and kits related to acadesine, or a prodrug, analog, or salt thereof, and / or a blood clotting inhibitor for preventing or reducing adverse side effects in a patient. The type of patient that may benefit includes a patient with decreased left ventricular function, a patient with a prior myocardial infarction, a patient undergoing non-vascular surgery, or a fetus during labor and delivery.
Owner:PERICOR THERAPEUTICS INC
Enalapril formulations
Provided herein are stable enalapril oral liquid formulations. Also provided herein are methods of using enalapril oral liquid formulations for the treatment of certain diseases including hypertension, heart failure and asymptomatic left ventricular dysfunction.
Owner:AZURITY PHARMA INC
Enalapril Formulations
Provided herein are stable enalapril oral liquid formulations. Also provided herein are methods of using enalapril oral liquid formulations for the treatment of certain diseases including hypertension, heart failure and asymptomatic left ventricular dysfunction.
Owner:AZURITY PHARMA INC
Methods of treating diabetic cardiomyopathy using glycogen phosphorylase inhibitors
The present invention provides methods of treating diabetic cardiomyopathy, the methods comprising administering to a patient having or at risk of having diabetic cardiomyopathy a therapeutically effective amount of a glycogen phosphorylase inhibitor. The present invention also provides methods of treating diabetic cardiomyopathy, the methods comprising administering to a patient having 1) diabetes and 2) having cardiovascular disease, ischemic heart disease, congestive heart failure, congestive heart failure but not having coronary arteriosclerosis, hypertension, diastolic blood pressure abnormalities, microvascular diabetic complications, abnormal left ventricular function, myocardial fibrosis, abnormal cardiac function, pulmonary congestion, small vessel disease, small vessel disease without atherosclerotic cardiovascular disease or luminal narrowing, coagulopathy, cardiac contusion, or having had or at risk of having a myocardial infarction a therapeutically effective amount of a glycogen phosphorylase inhibitor.
Owner:PFIZER INC
Method and system for use in monitoring left ventricular dysfunction
Owner:N I MEDICAL
Use of organic compounds
The invention relates to a method of treating cardiovascular disease and inducing cardiovascular remodeling and thereby reducing the risk of morbidity, especially stroke, and mortality following a MI, especially MI complicated with left ventricular dysfunction or heart failure, through the administration of an ARB, particularly valsartan and an ACE inhibitor, particularly captopril. The present invention also discloses dosing regimens and dosage packs for the above treatments.
Owner:GRAVES KURT CHUM
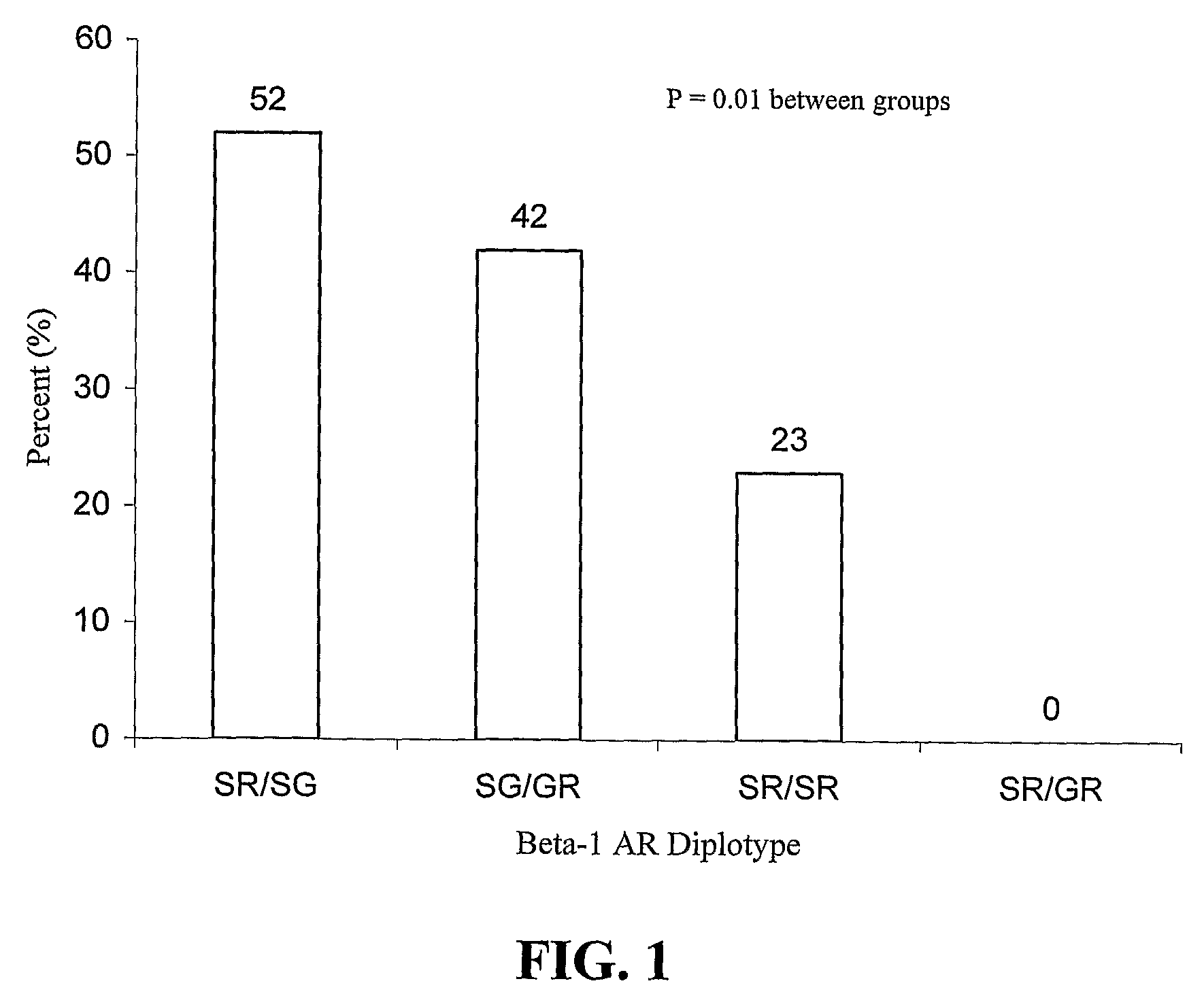
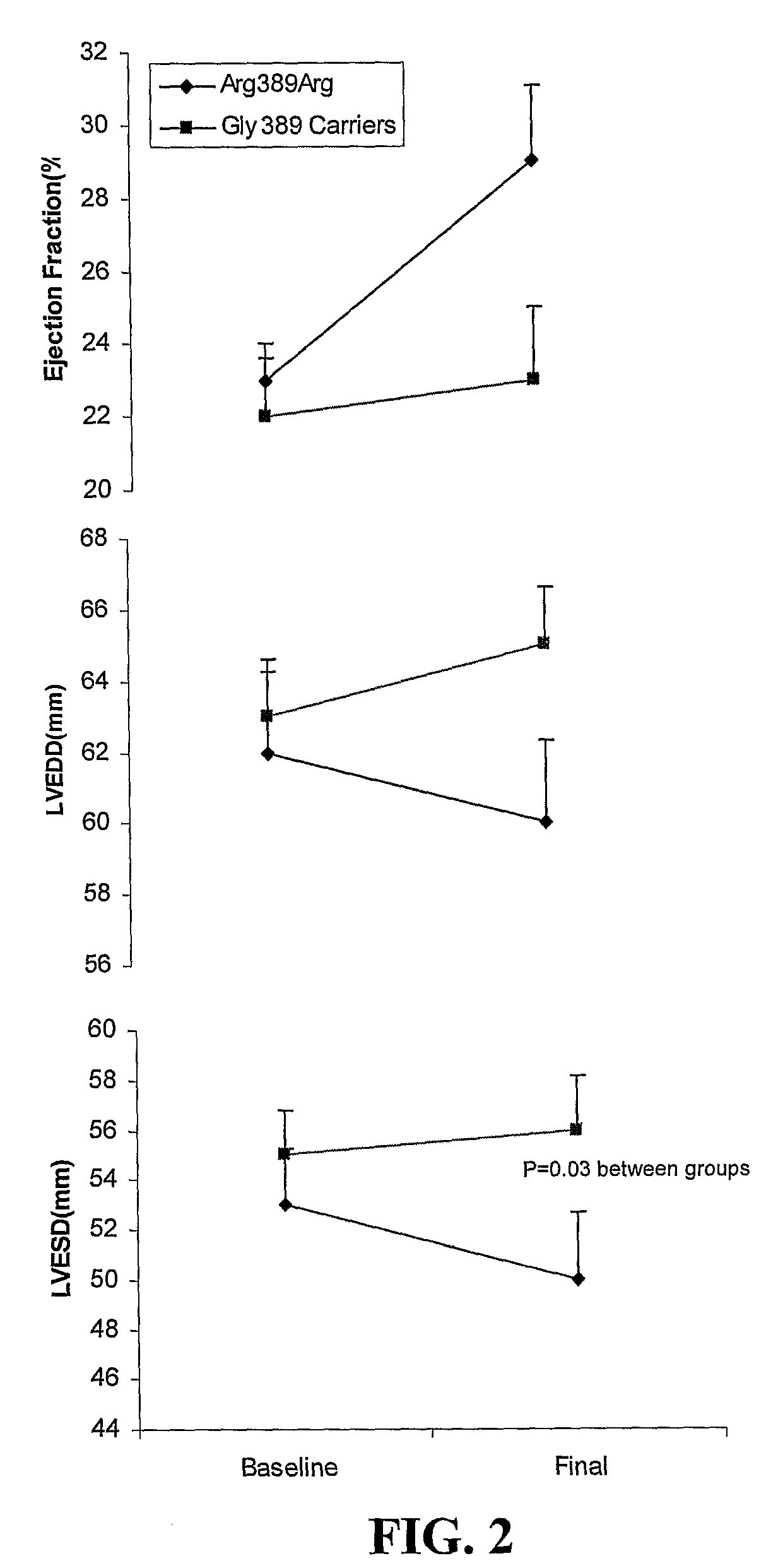
B-Blocker Pharmacogenetics in Heart Failure
InactiveUS20080269346A1Reduce riskPromote absorptionOrganic active ingredientsBiocidePharmacogeneticsTolerability
Polymorphisms in the beta-1 adrenergic receptor that (1) affect tolerability to beta-blocker treatment or (2) influence responsiveness to beta-blocker treatment are disclosed. Additionally, methods of predicting that an individual would obtain clinical improvement in left ventricular function as a result of β-blocker therapy or identifying at least one individual that would obtain a clinical improvement in left ventricular function as a result of β-blocker therapy are provided. Methods of treating patients characterized by such polymorphisms are also illustrated.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
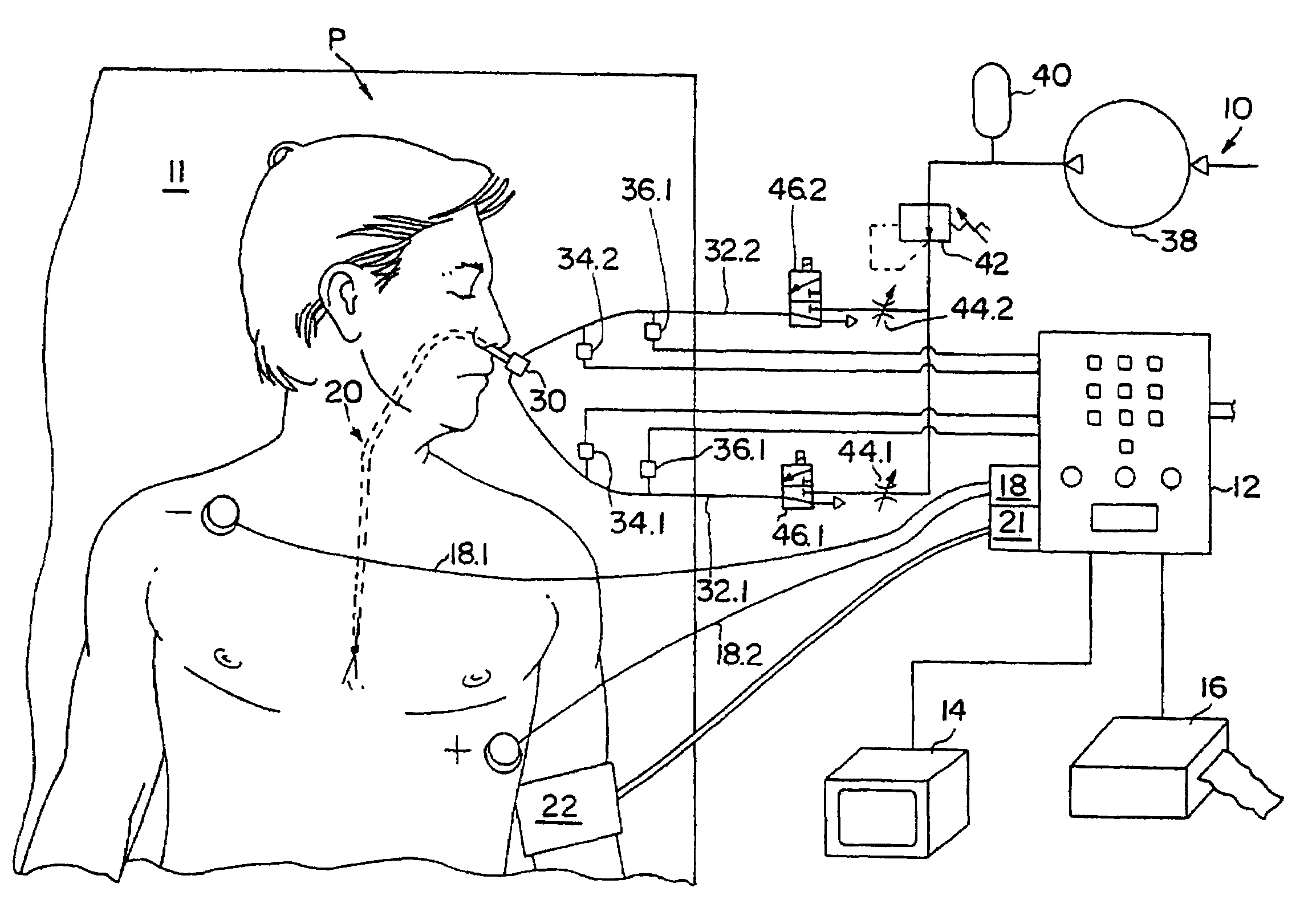
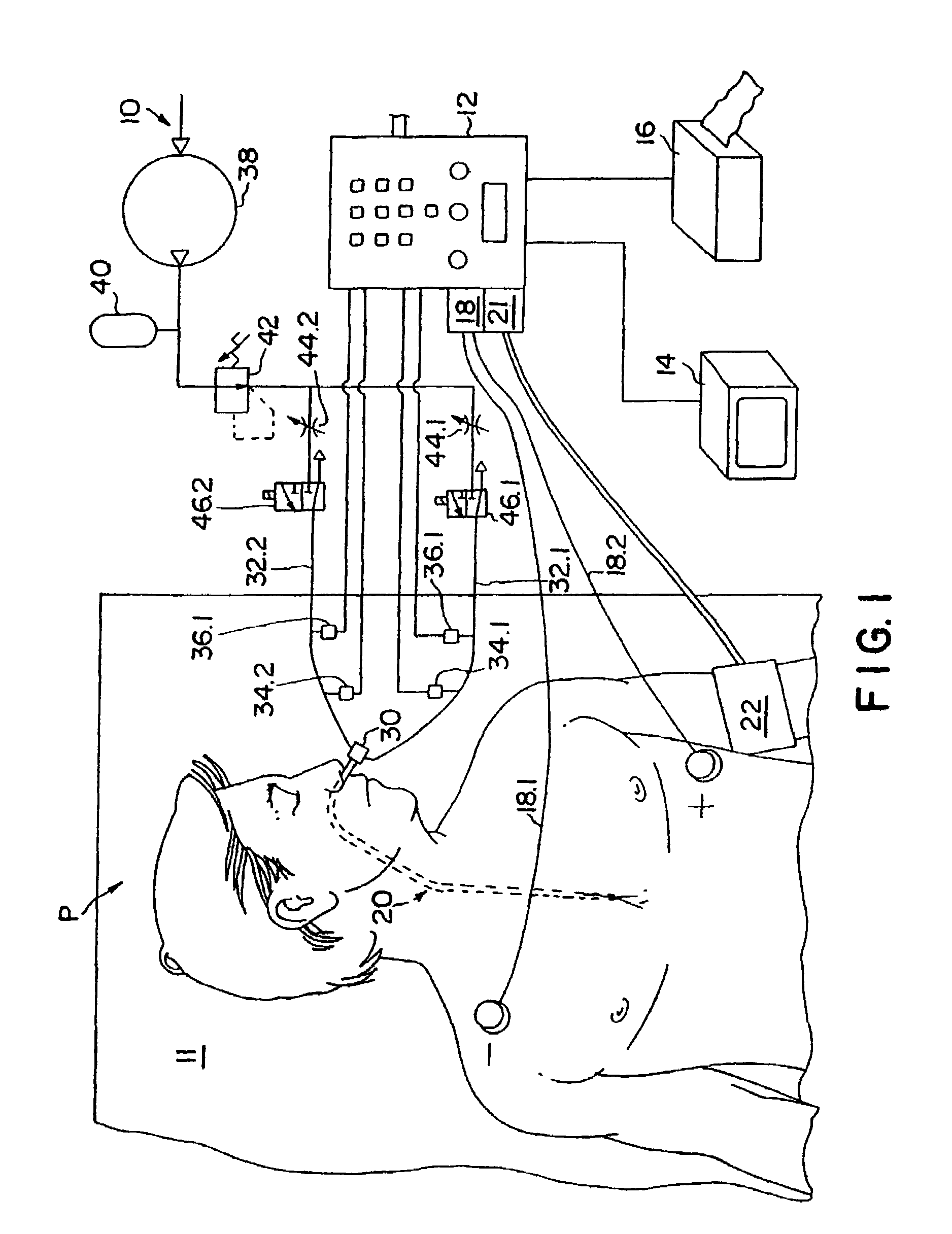
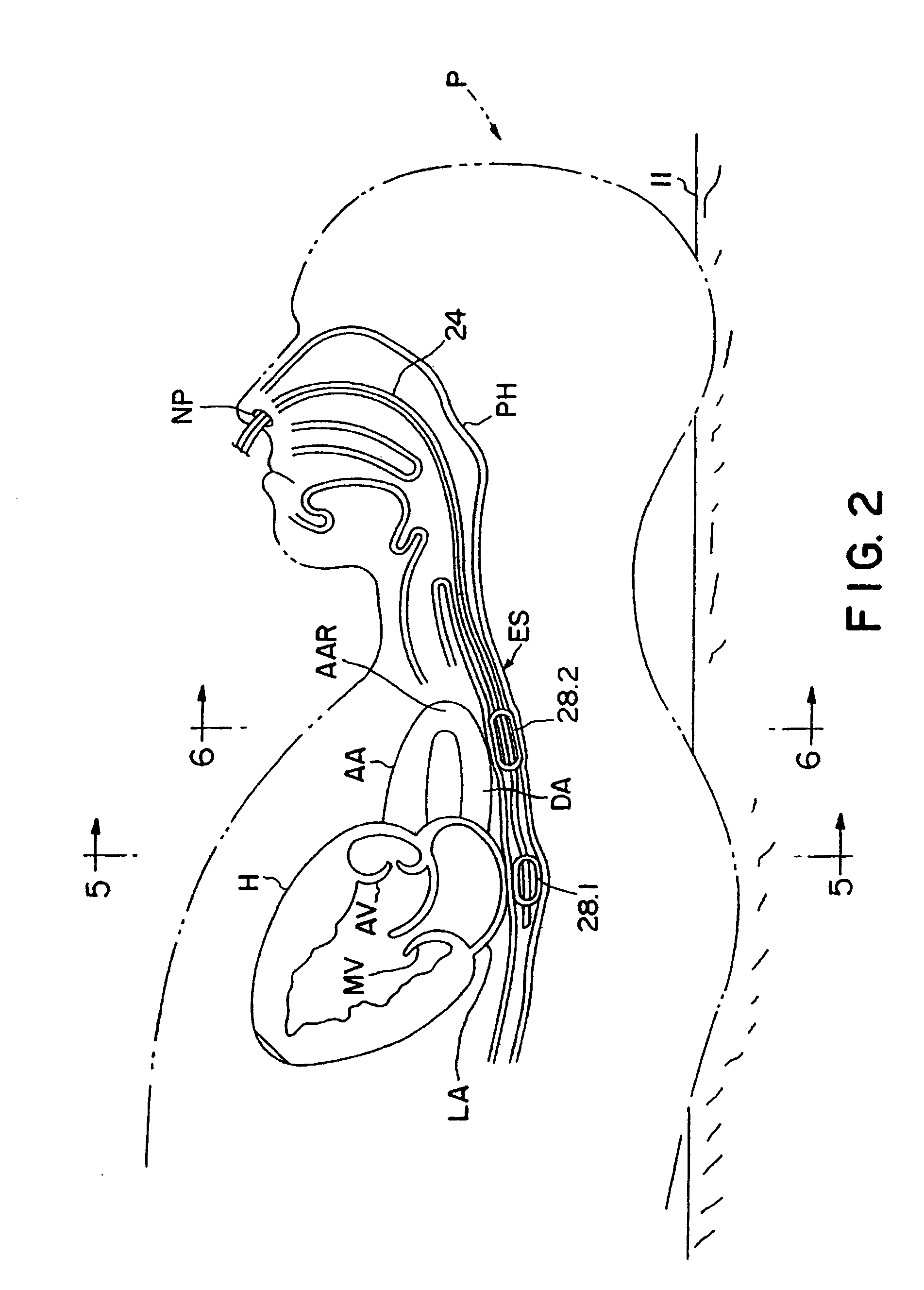
Method of determining cardiac indicators
A system and method for determining indicators of left ventricular function, and for determining the effectiveness of a cardiac therapy are disclosed. The indicators of left ventricular function can include isovolumetric relaxation time and the negative slope of a V wave obtained from measuring changes in left atrial pressure during diastole. The apparatus may include a catheter comprising an inflatable balloon for insertion into the esophagus of an individual, a compressor device for pressurizing the balloon, and a means for sensing and recording pressure changes on the balloon.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Novel reverse arterial intubation tube
ActiveCN102553058AIncrease blood oxygen supplyIncrease blood supply efficiencyCatheterCoatingsIliac arteryGuide wires
A novel reverse arterial intubation tube relates to the technical field of medical instruments and comprises a catheter with a hollow inner cavity. The novel reverse arterial intubation tube is characterized in that the catheter is an 'S'-shaped spiral structure, a guide wire inlet is arranged at a head end of the catheter, an outlet of the catheter is spiral, the flowing direction of the outlet of the catheter is opposite to the flowing direction of an inlet, a spiral sheet is arranged in the inner cavity nearby the outlet of the catheter, the inner cavity of the catheter is divided into a left branched blood flow pipe and a right branched blood flow pipe by the spiral sheet, the branched blood flow pipe on the right side is gradually reduced and closed, a side opening is arranged on the surface on the right side of the catheter, and a lubricating coating is coated on the surface of the catheter. The novel reverse arterial intubation tube not only can increase blood oxygen supply for upper limbs and the head of a patient, increases blood supply efficiency of forward blood flow of the artery of the patient and reduces influences of reverse blood flow to functions of the coronary artery, the aortic valve and the left ventricle of the patient as much as possible, but also can avoid the problem that blood supply for tissues of lower limbs of the patient is excessive relatively, and avoids or reduces 'south-north syndrome'.
Owner:ANHUI TONGLING BIONIC TECH CO LTD
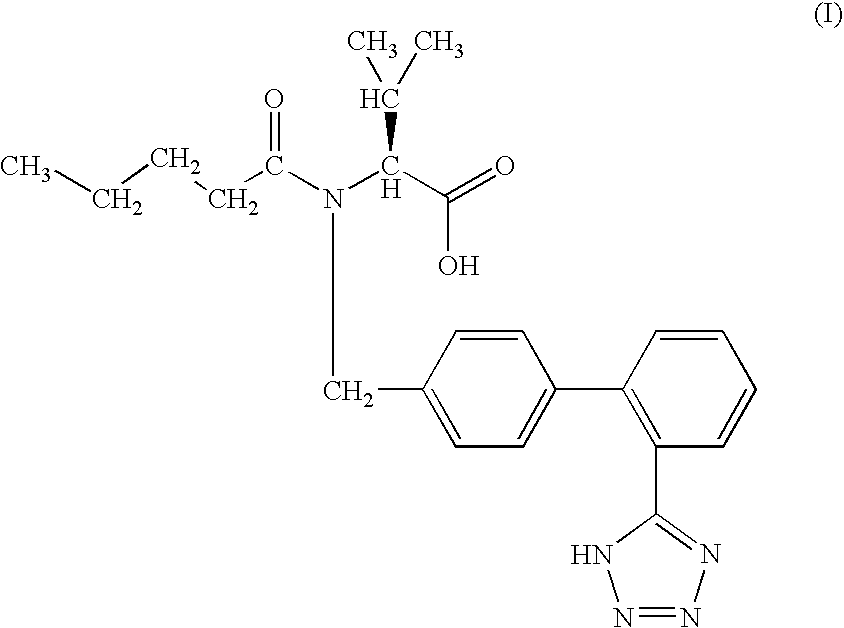
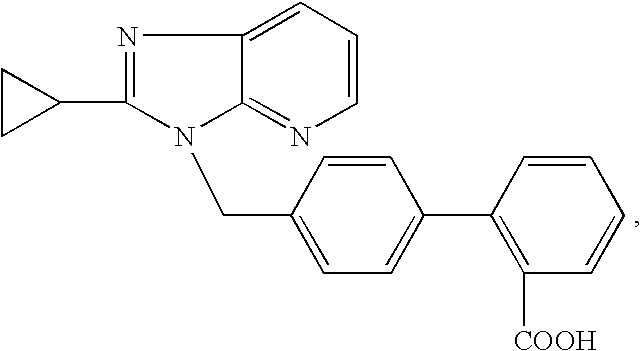
Modulators of the beta-3 adrenergic receptor useful for the treatment or prevention of disorders related thereto
InactiveUS20190284200A1Inhibition of contractilityImproves contractile functionOrganic active ingredientsOrganic chemistryLeft ventricular sizeKidney
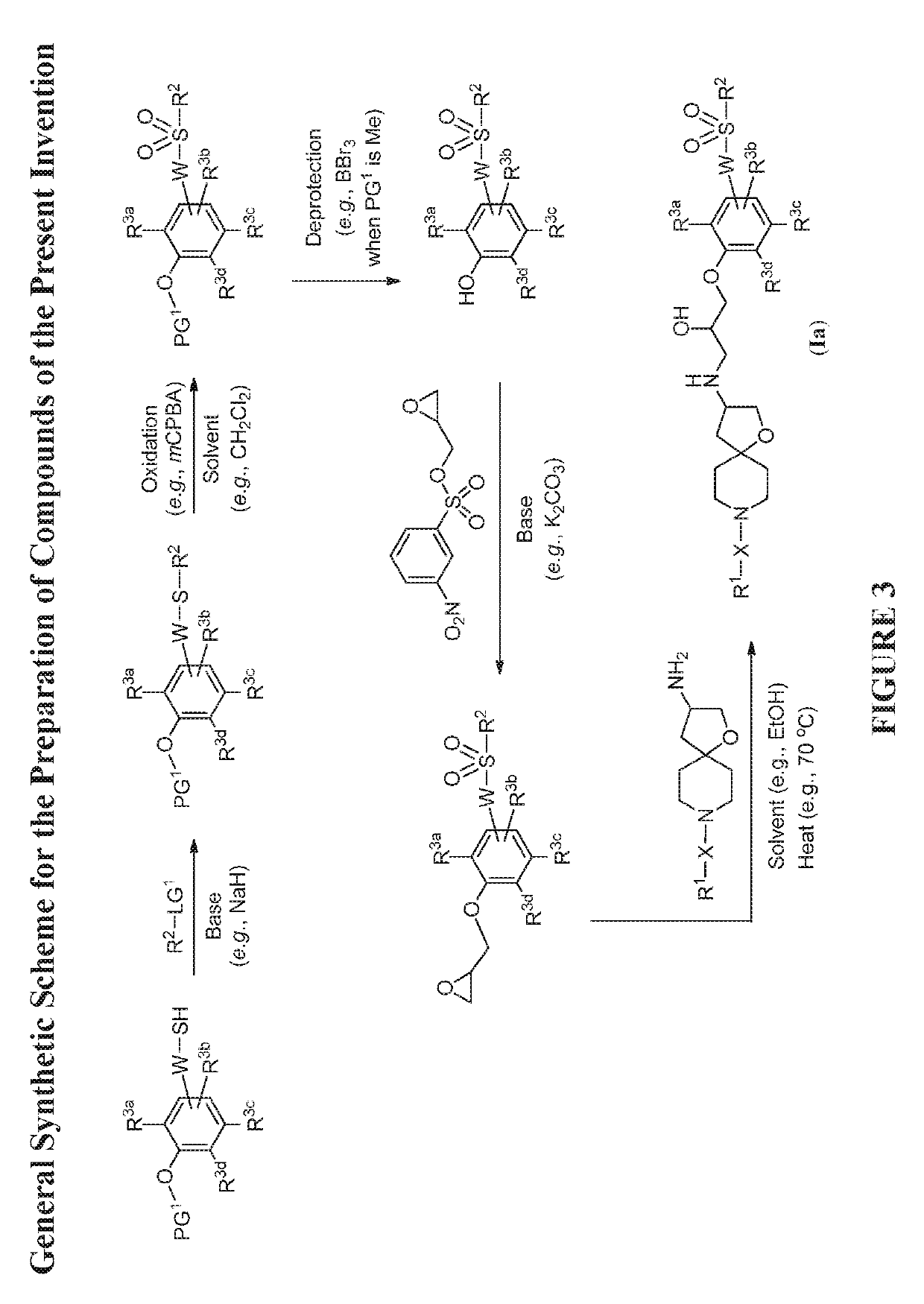
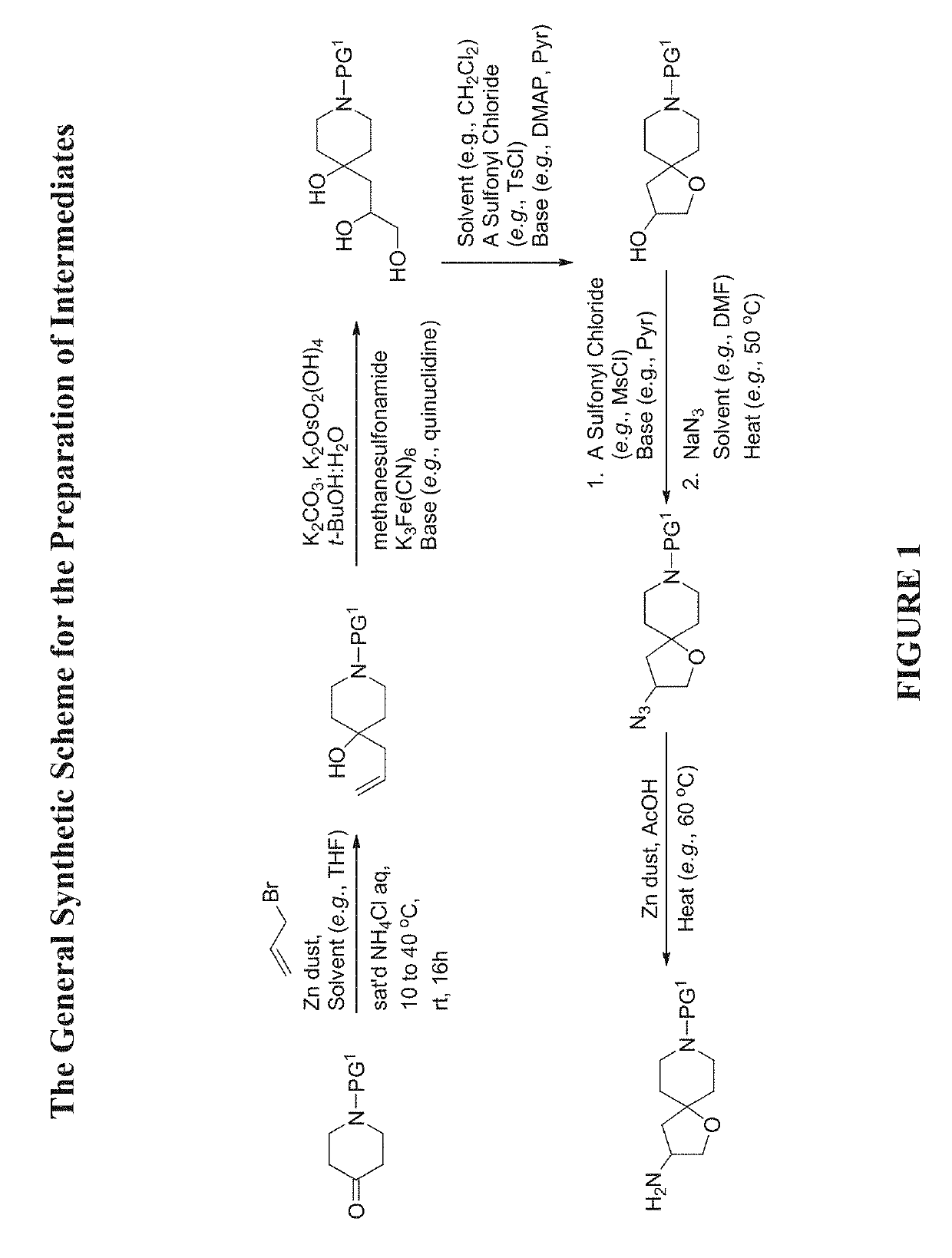
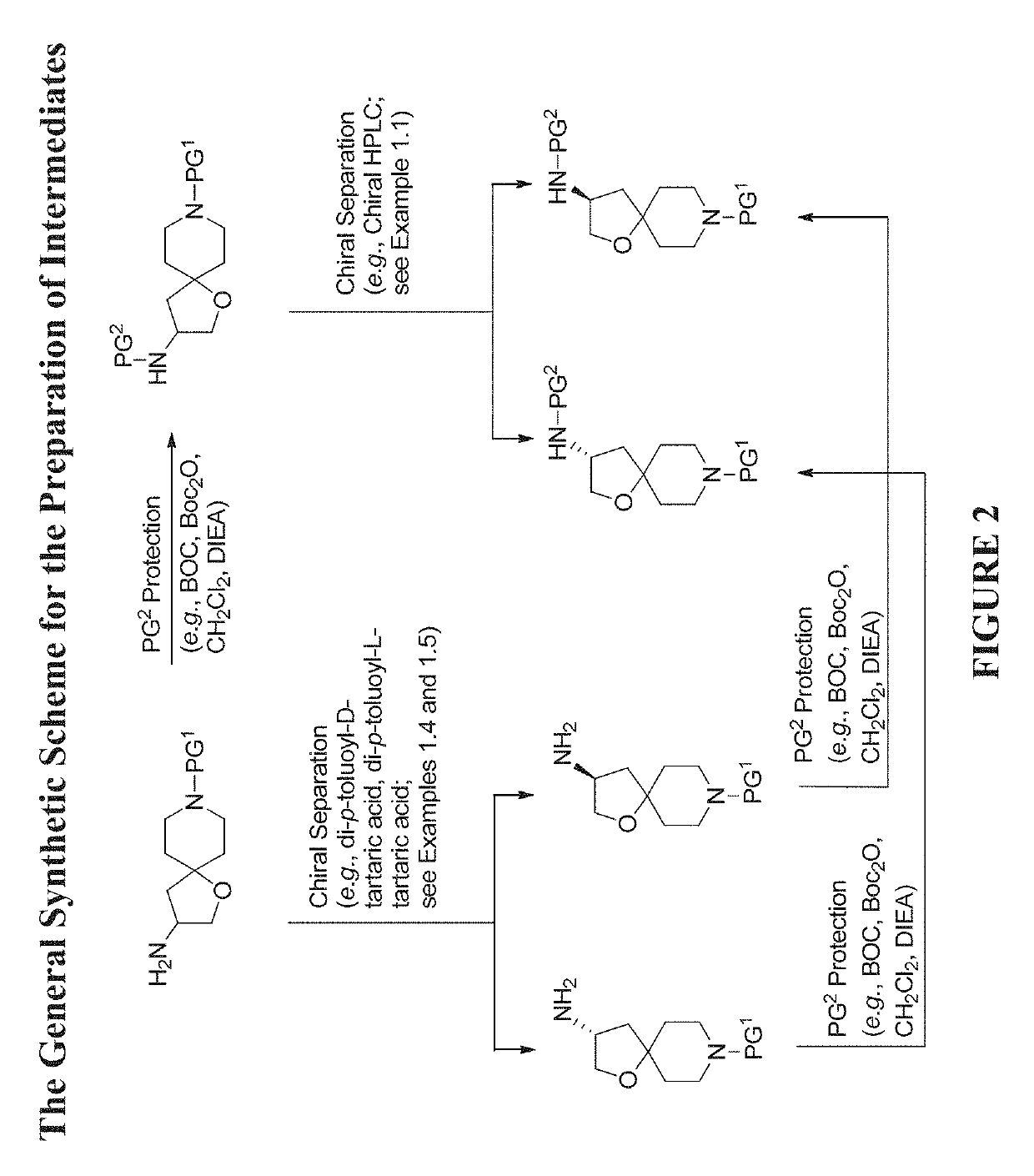
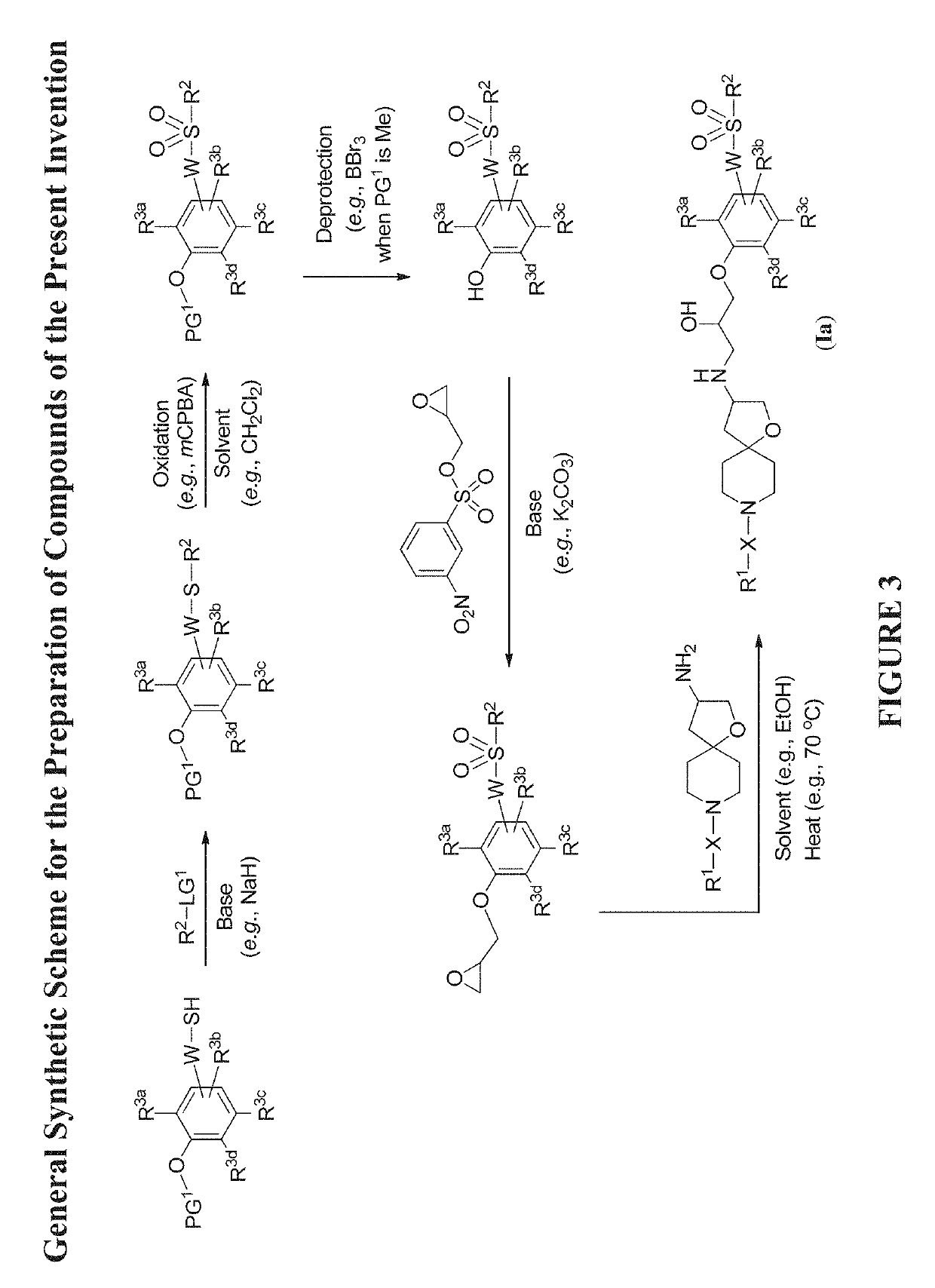
The present invention relates to compounds of Formula (Ia) and pharmaceutical compositions thereof that modulate the activity of the beta-3 adrenergic receptor. Compounds of the present invention and pharmaceutical compositions thereof are directed to methods useful in the treatment of a beta-3 adrenergic receptor-mediated disorder, such as, heart failure; cardiac performance in heart failure; mortality, reinfarction, and / or hospitalization in connection with heart failure; acute heart failure; acute decompensated heart failure; congestive heart failure; severe congestive heart failure; organ damage associated with heart failure (e.g., kidney damage or failure, heart valve problems, heart rhythm problems, and / or liver damage); heart failure due to left ventricular dysfunction; heart failure with normal ejection fraction; cardiovascular mortality following myocardial infarction; cardiovascular mortality in patients with left ventricular failure or left ventricular dysfunction; left ventricular failure; left ventricular dysfunction; class II heart failure using the New York Heart Association (NYHA) classification system; class III heart failure using the New York Heart Association (NYHA) classification system; class IV heart failure using the New York Heart Association (NYHA) classification system; LVEF<40% by radionuclide ventriculography; LVEF≤35% by echocardiography or ventricular contrast angiography; and conditions related thereto.
Owner:ARENA PHARMA
Modulators of the beta-3 adrenergic receptor useful for the treatment or prevention of disorders related thereto
ActiveUS20190292196A1Lower performance requirementsImproves contractile function and hemodynamic statusOrganic active ingredientsOrganic chemistryLeft ventricular sizeKidney
The present invention relates to compounds of Formula (Ia) and pharmaceutical compositions thereof that modulate the activity of the beta-3 adrenergic receptor. Compounds of the present invention and pharmaceutical compositions thereof are directed to methods useful in the treatment of a beta-3 adrenergic receptor-mediated disorder, such as, heart failure; cardiac performance in heart failure; mortality, reinfarction, and / or hospitalization in connection with heart failure; acute heart failure; acute decompensated heart failure; congestive heart failure; severe congestive heart failure; organ damage associated with heart failure (e.g., kidney damage or failure, heart valve problems, heart rhythm problems, and / or liver damage); heart failure due to left ventricular dysfunction; heart failure with normal ejection fraction; cardiovascular mortality following myocardial infarction; cardiovascular mortality in patients with left ventricular failure or left ventricular dysfunction; left ventricular failure; left ventricular dysfunction; class II heart failure using the New York Heart Association (NYHA) classification system; class III heart failure using the New York Heart Association (NYHA) classification system; class IV heart failure using the New York Heart Association (NYHA) classification system; LVEF<40% by radionuclide ventriculography; LVEF≤35% by echocardiography or ventricular contrast angiography; and conditions related thereto.
Owner:ARENA PHARMA
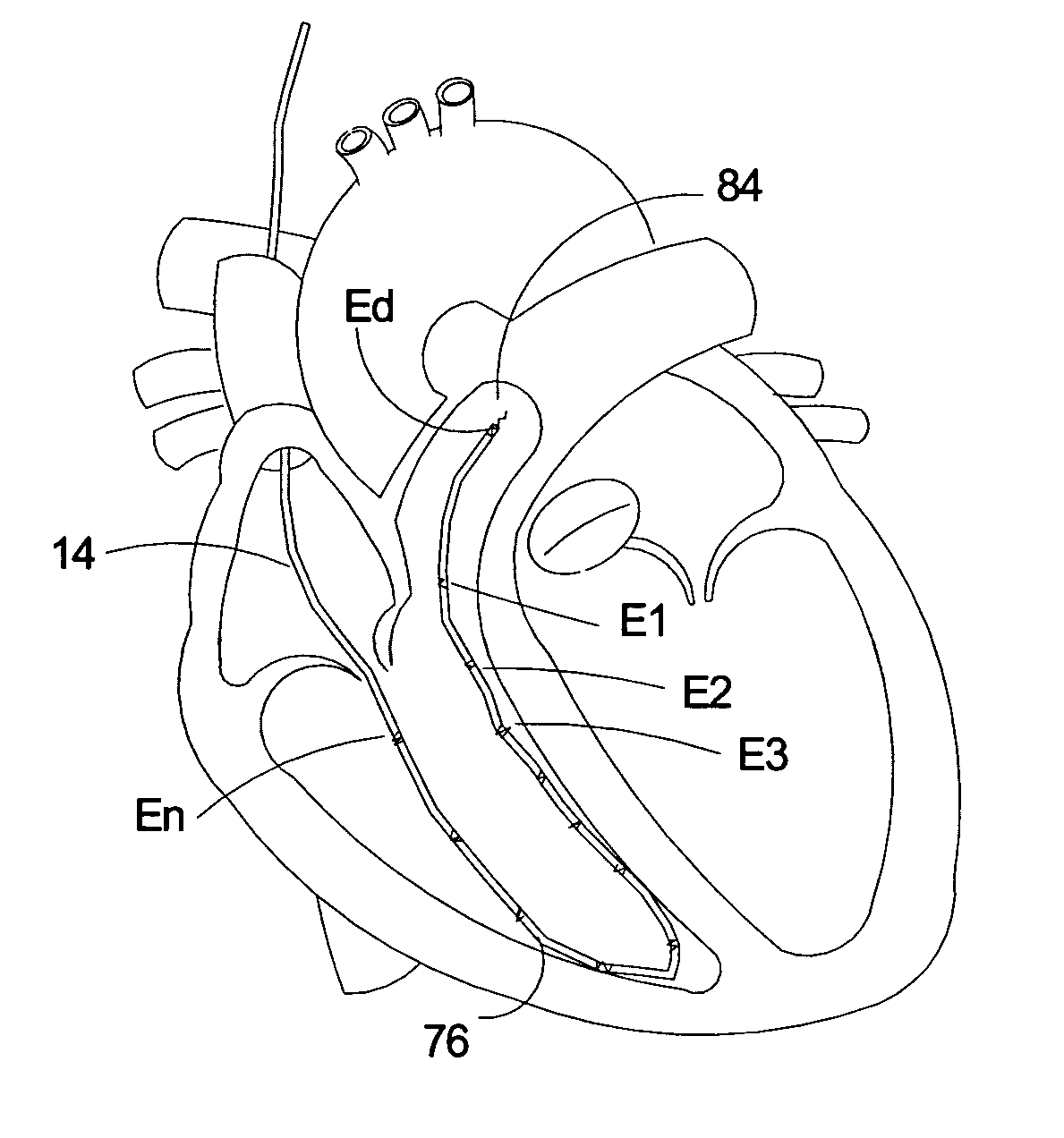
Monitoring QRS complex to identify left ventricular dysfunction
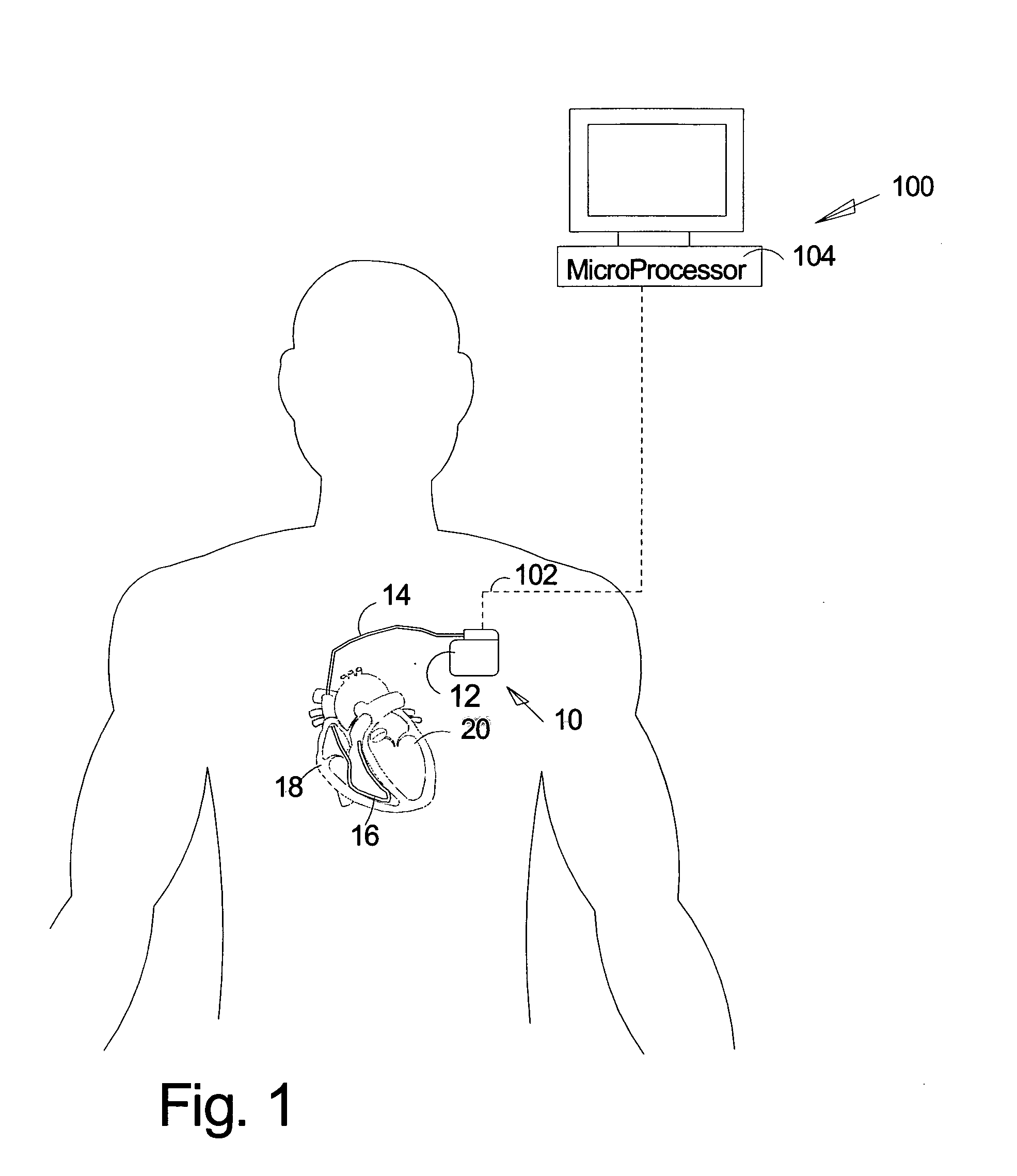
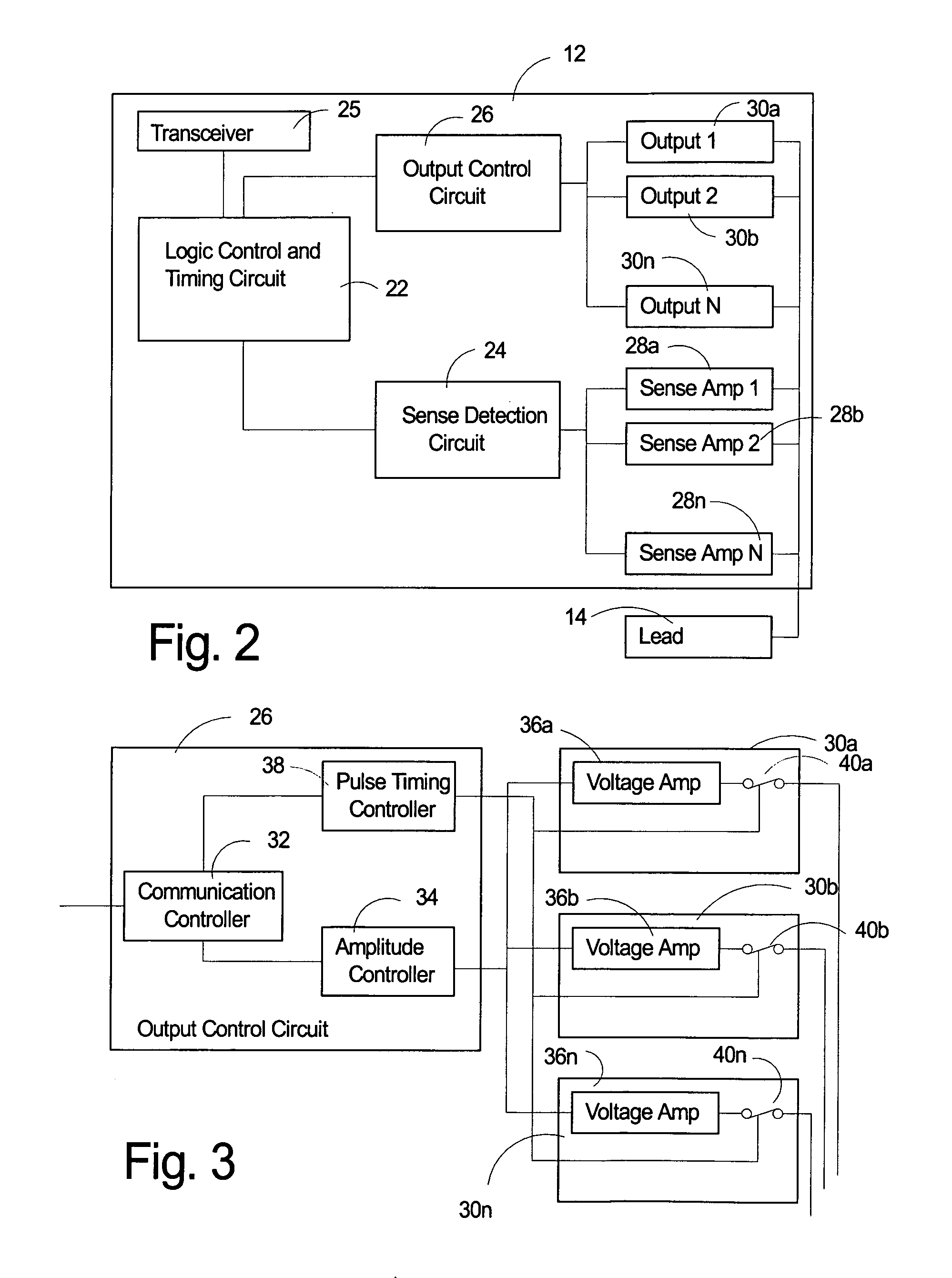
ActiveUS7930026B2Quick checkElectrocardiographyHeart stimulatorsLeft ventricular sizeLinksventrikulare funktion
An implantable medical device identifies onset and / or progression of left ventricular dysfunction (LVD). The implantable medical device receives a signal that represents electrical activity within a heart, e.g., an electrogram signal, and digitally processes the signal to assess left ventricular function. In particular, the implantable medical device measures at least one characteristic of QRS complexes within the signal, and assesses left ventricular function based on measurements. The implantable medical device may, for example, measure the width of the QRS complexes and / or the amplitude of the R-waves within the QRS complexes. The implantable medical device may alert a patient or clinician of the onset or progression of LVD, and may control delivery of therapies, such as rate-responsive pacing and cardiac resynchronization pacing, based on the measurements. The implantable medical device may also control delivery of a drug by an implanted drug delivery device, e.g., drug pump, based on the measurements.
Owner:MEDTRONIC INC
External counter pulsation treatment
ActiveUS20090247809A1Effective treatmentEfficient managementPneumatic massageHeart stimulatorsButtocksLeft ventricular size
A method for treating patients suffering from left ventricular dysfunction is disclosed. The method involves applying, during diastole, for a time period of about one hour, at least five days each week for at least about six weeks, an incrementally increasing external therapeutic pressure sequentially to the patients' lower extremities from first the calves, then the thighs and last the buttocks. The initial hourly treatments are carried out at a peak diastolic / systolic pressure ratio (D / S Ratio) in the range of about 0.4:1 up to about 0.9:1, depending on the patient's left ventricular ejection fraction. The D / S Ratio is increased slightly during the next set of hourly treatments, the D / S Ratio is again increased slightly during the next following set of hourly treatments, the D / S Ratio is again increased slightly during the next set of hourly treatments, and finally the D / S Ratio is increased slightly and maintained during the remaining set of hourly treatments. The patient's cardiopulmonary functions preferably are monitored to determine if additional external therapeutic pressure treatments are needed.
Owner:CARDIOMAX
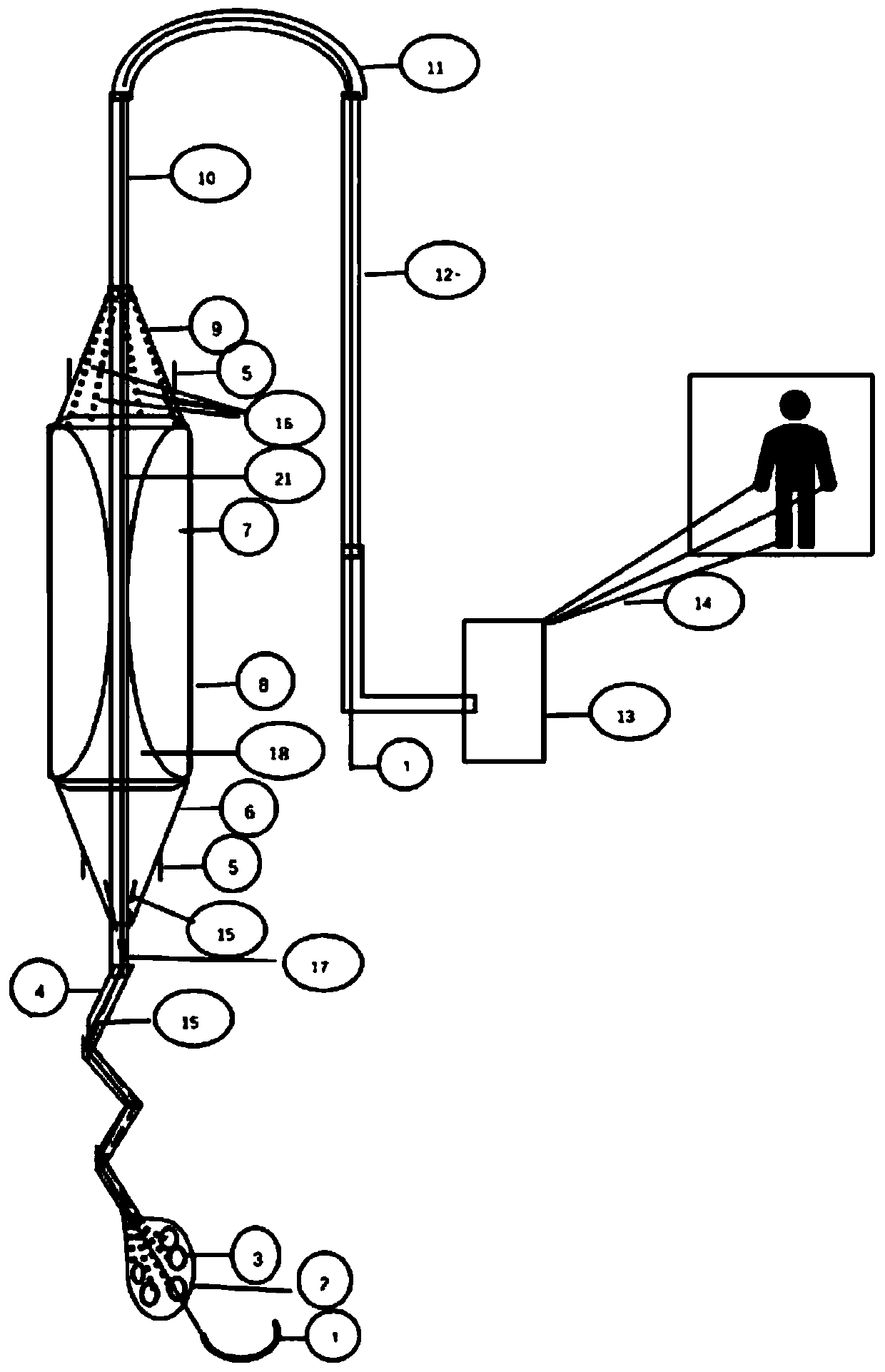
Intractable heart failure left ventricular function auxiliary device
PendingCN110124133AReduce mortalityElectrocardiographyMedical devicesHeart transplantationLeft ventricular size
The invention discloses an intractable heart failure left ventricular function auxiliary device. Various straws and cavity tunnels are designed in a suction head, outer sucking holes are formed in thelower wall and the outer side of the suction head, a straw is led to a proximal end conical cavity inwards, a one-way valve is designed at a position communicated with the cavity in a pipeline of thestraw, the straw is communicated with an inner cavity of an air bag through the one-way valve, a single channel is connected with the drop-shaped suction head in a straw valve crossing section, a distal end is communicated with an air bag chamber, four one-way valves are arranged on the outer wall of the trapezoid side surface of a capsule and communicated with an inner cavity of the air bag chamber through channels, conical upper end is connected with an air-space tunnel through a trachea, branch tracheae pass the air bag chamber downwards and are opened on the inner side of the wall of theair bag and converged into a main trachea upwards, the main trachea is communicated with outside thought an artery, and the lower end of a guide wire is communicated with the drop-shaped suction head.The device has the advantages that the device can continuously work under the condition of left ventricular function loss, and time is bought for clinical rescue or heart transplantation.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
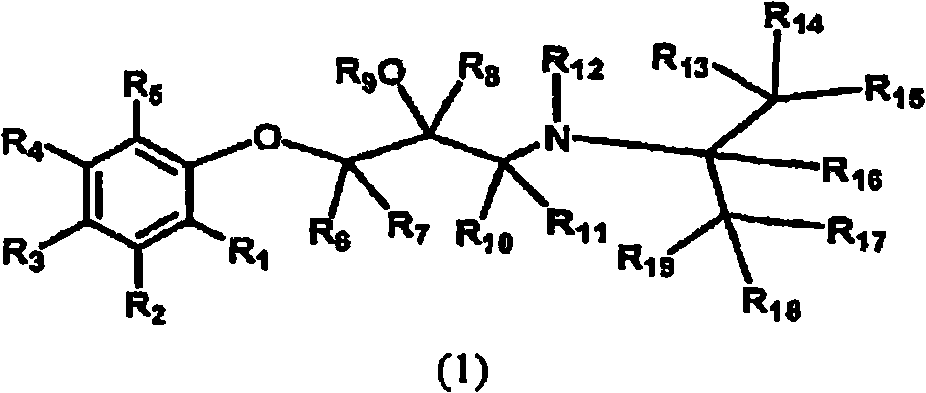
Deuterated aminoglycidyl compounds
InactiveCN101528669AOrganic active ingredientsIsotope introduction to organic compoundsLeft ventricular sizeChronic stable angina
The present invention provides substituted aminoglycidyl compounds of Formula (1), processes of preparation, and pharmaceutical compositions thereof and methods of their use for treating, preventing, or ameliorating one or more symptoms of a social anxiety disorder, an anxiety disorder, hyperthyroidism, tremor, glaucoma, hypertension, coronary artery bypass graft, chronic stable angina, atrial arrhythmia, migraine, bleeding esophageal varices, hypertrophic subaortic stenosis, heart failure, post-myocardial infarction, decreased left ventricular function after recent myocardial infarction, and / or any disorder ameliorated by beta adrenergic receptor modulators.
Owner:AUSPEX PHARMA INC
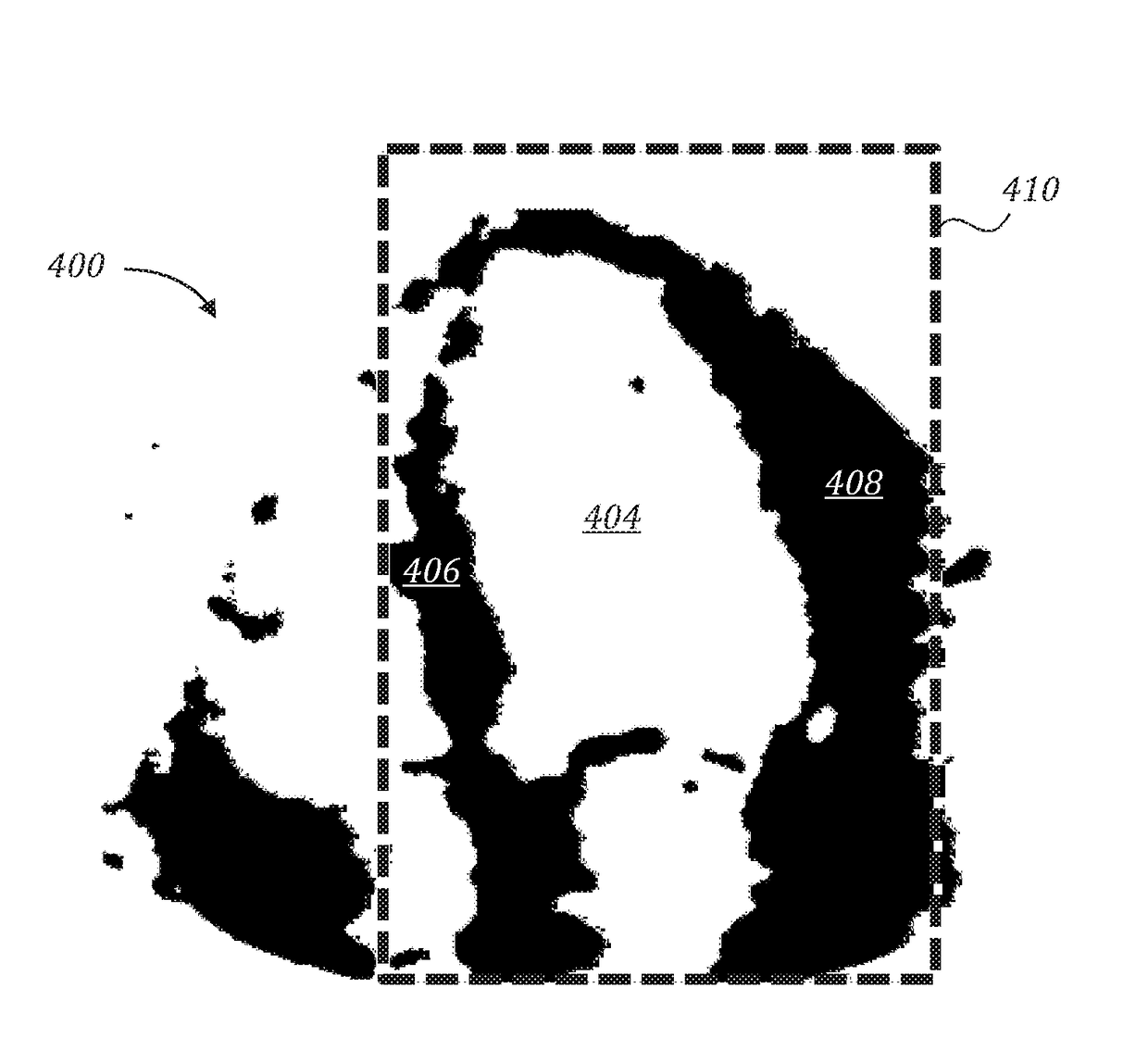
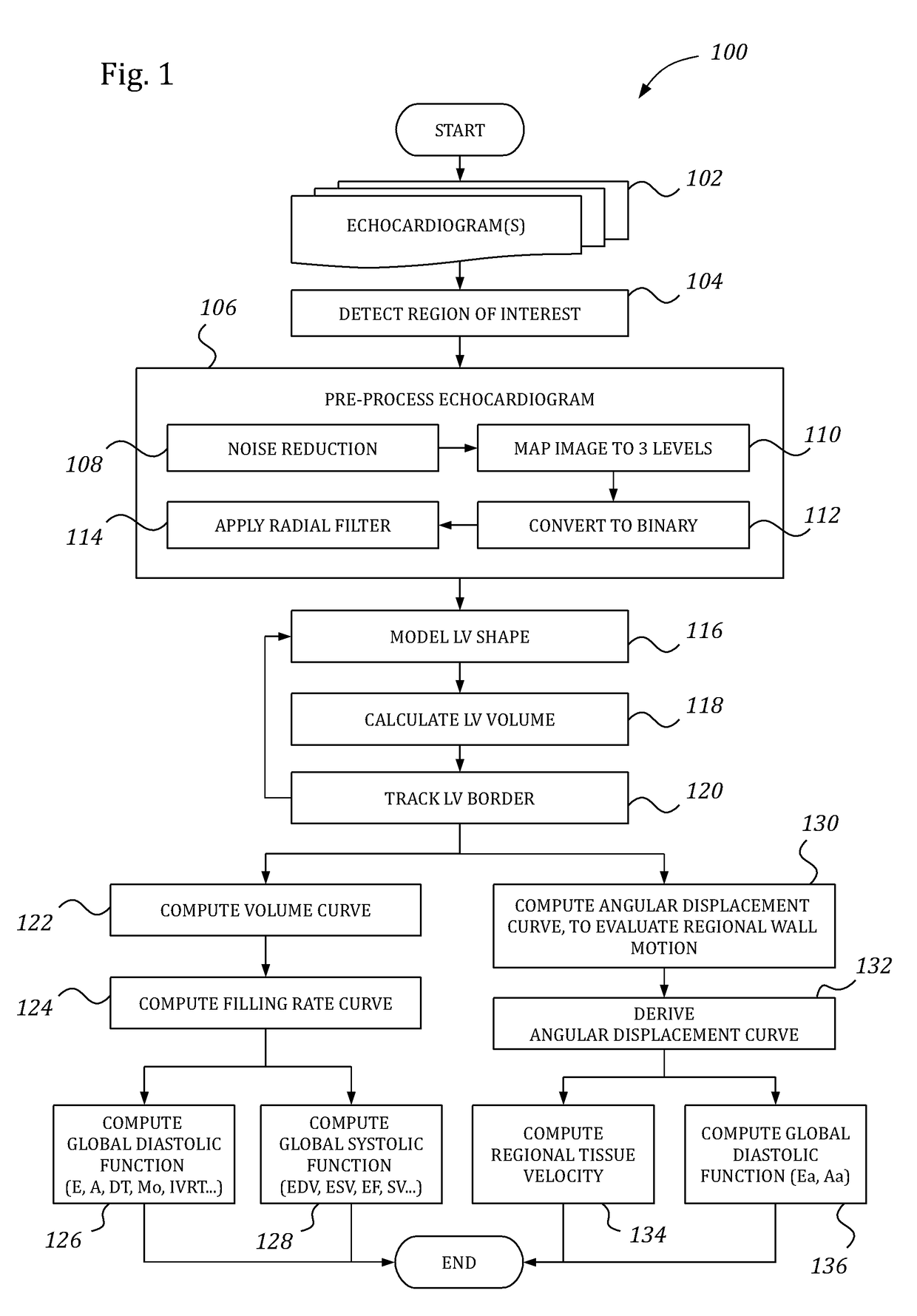
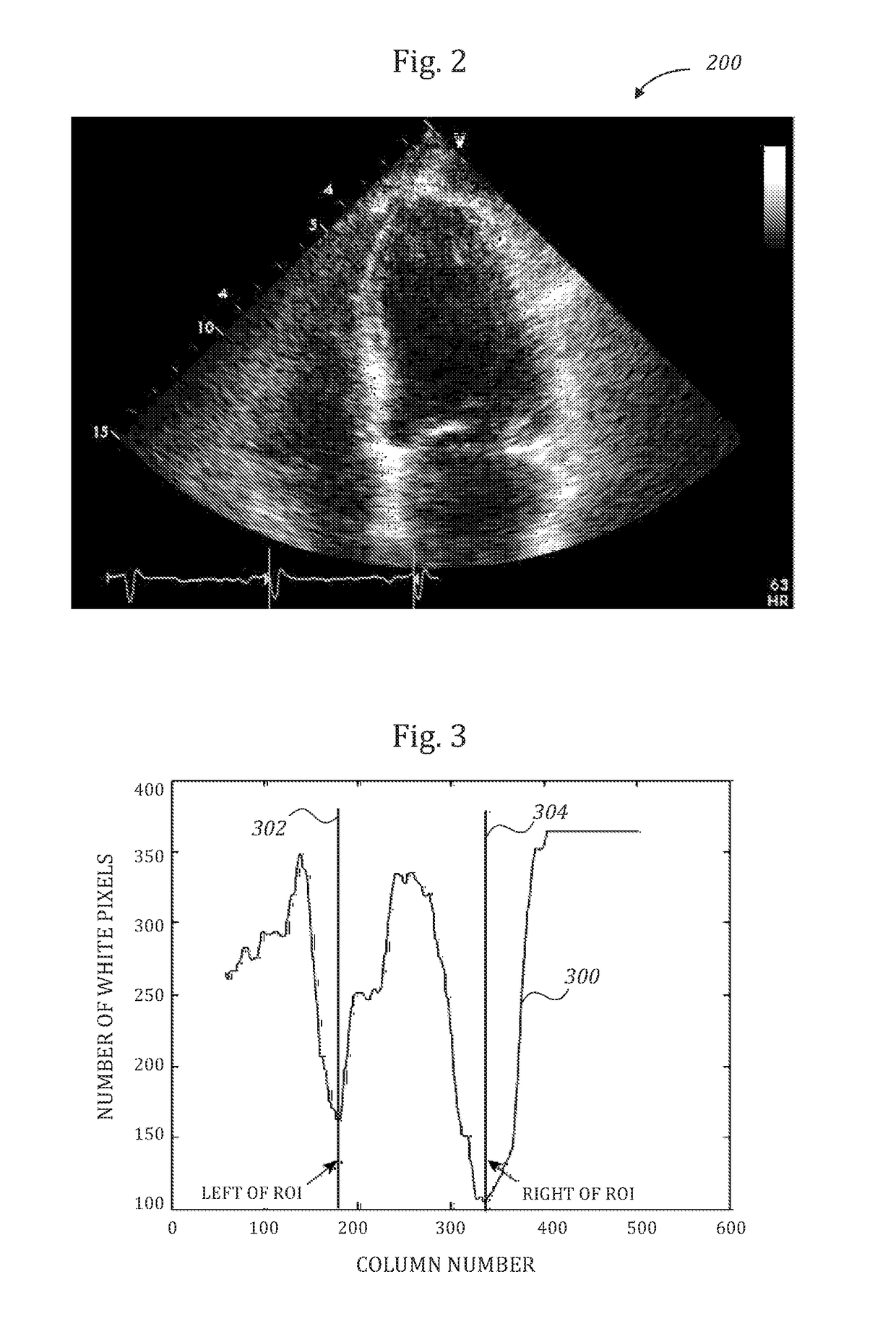
Automatic left ventricular function evaluation
ActiveUS10078893B2Reduce noiseImage enhancementImage analysisLeft ventricular sizeLinksventrikulare funktion
Owner:DIACARDIO
Methods, compositions, and formulations for preventing or reducing adverse effects in a patient
The present invention provides methods, compositions, formulations, and kits related to acadesine, or a prodrug, analog, or salt thereof, and / or a blood clotting inhibitor for preventing or reducing adverse side effects in a patient. The type of patient that may benefit includes a patient with decreased left ventricular function, a patient with a prior myocardial infarction, a patient undergoing non-vascular surgery, or a fetus during labor and delivery.
Owner:PERICOR THERAPEUTICS INC
External counter pulsation treatment
A method for treating patients suffering from left ventricular dysfunction is disclosed. The method involves applying, during diastole, for a time period of about one hour, at least five days each week for at least about six weeks, an incrementally increasing external therapeutic pressure sequentially to the patients' lower extremities from first the calves, then the thighs and last the buttocks. The initial hourly treatments are carried out at a peak diastolic / systolic pressure ratio (D / S Ratio) in the range of about 0.4:1 up to about 0.9:1, depending on the patient's left ventricular ejection fraction. The D / S Ratio is increased slightly during the next set of hourly treatments, the D / S Ratio is again increased slightly during the next following set of hourly treatments, the D / S Ratio is again increased slightly during the next set of hourly treatments, and finally the D / S Ratio is increased slightly and maintained during the remaining set of hourly treatments. The patient's cardiopulmonary functions preferably are monitored to determine if additional external therapeutic pressure treatments are needed.
Owner:CARDIOMAX
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com