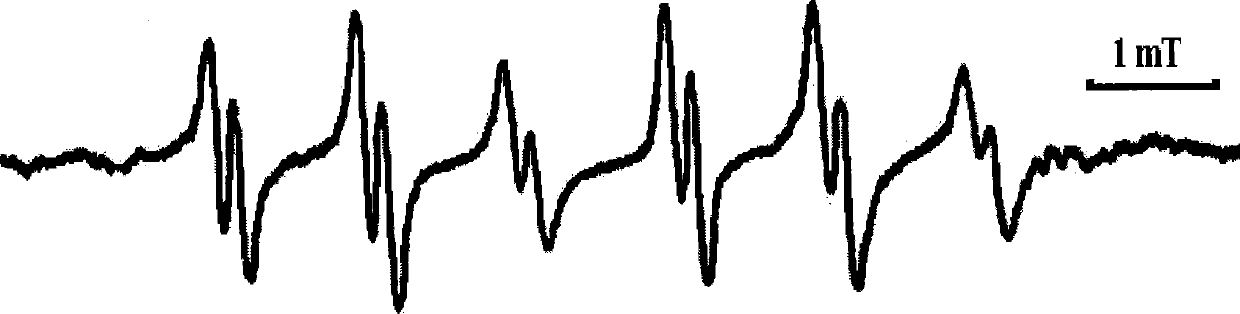
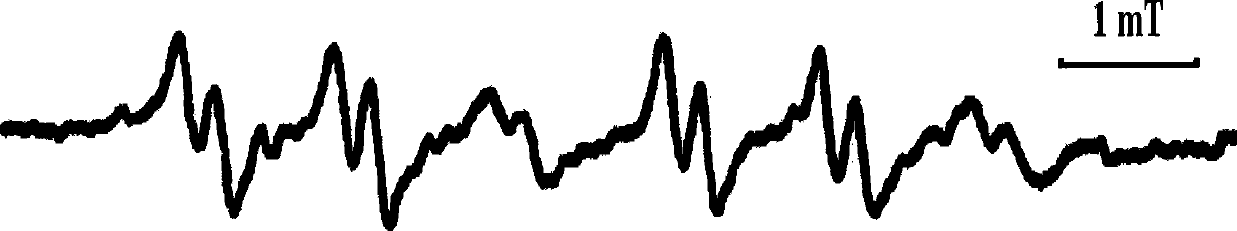
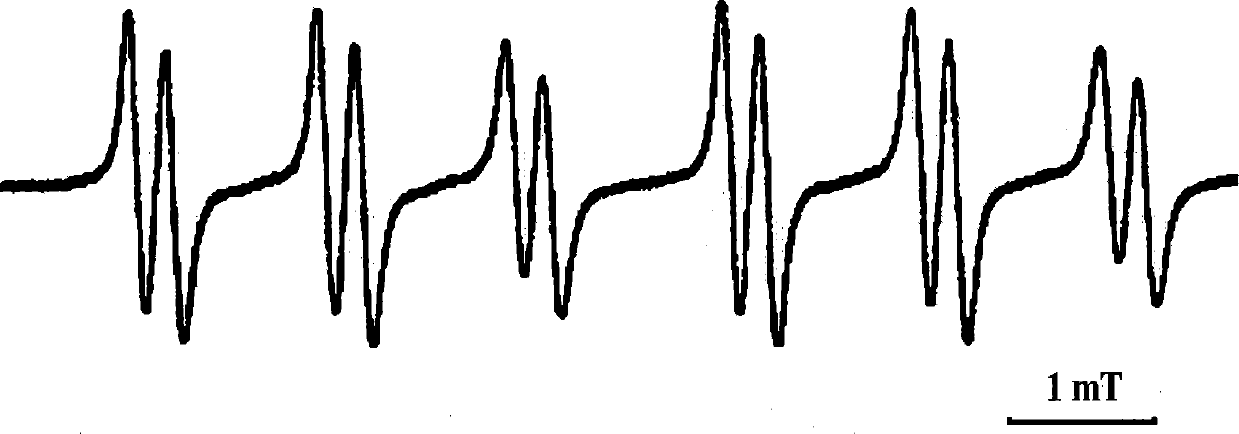
Nitrones with the function of trapping free radicals and their preparation methods and applications
A technology of compounds and free radicals, applied in peptides, material excitation analysis, fluorescence/phosphorescence, etc., can solve the problems of small molecule free radicals chemical instability, low detection method, short lifespan, etc., and achieve high-efficiency free radical capture ability, Effects of improving analytical sensitivity and reducing quenching probability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1. Preparation of glutathione-linked phosphoryl radical capture probe (compound III)
[0057] 1. Preparation of diethyl phosphite (compound 1)
[0058] A 500mL dry two-necked flask with a built-in magnetic stirrer is used as the reactor, one is connected to a constant pressure dropping funnel, and the other is connected to a thermometer. A mixture of 88 mL (1.5 mol, 70 g) of absolutely absolute ethanol (purity> 99.95%) and 50 mL of anhydrous benzene was added to the reactor. It was cooled to 10°C in an ice water bath, and a mixture consisting of 44 mL (0.5 mol, 69 g) of newly distilled phosphorus trichloride and 50 mL of anhydrous benzene was added dropwise over 1 hour under stirring. The reaction temperature is controlled between 5-10°C. After the reaction is completed, the water pump is depressurized, and nitrogen is introduced from the capillary tube to eliminate hydrogen chloride. Distill the solvent and remaining reactants (15-20°C), and then use an oil pump ...
Embodiment 2
[0086] Example 2. Preparation of phosphoryl radical trap probes linking glutathione and fluorescein isothiocyanate (IV)
[0087] Configure pH 9.5, 500mM carbonate buffer solution (add 17g Na in 1L ultrapure water 2 CO 3 And 28gNaHCO 3 ). The FITC (1mg / ml, 3.7g, 0.0095mM) carbonate buffer solution was dropped into the compound III (1mg / ml, 6.3mg, 0.0095mM) under agitation at a temperature of 4℃ under dark conditions. In the carbonate buffer solution, the reaction was carried out in a 25ml Erlenmeyer flask. After 5 minutes of feeding was completed, the stirring was continued for 12 hours. To ensure the temperature, the reactor was moved into a 4°C refrigerator.
[0088] After the reaction was completed, Sephadex G-10 was used for desalination, and the final product was directly separated by HPLC. The product structure was identified by mass spectrometry. MS(ESI, m / z): 1065.3([M+H] + , 100%), 1103.3([M+K] + , 32.1%).
[0089]
[0090] Compound (IV)
Embodiment 3
[0091] Example 3. Preparation of phosphoryl radical capture probe linking thymopentin and dansyl chloride fluorescein (VI)
[0092] See Example 1 for the synthesis steps of compound 7.
[0093] 1. Link thymopentin and phosphoryl radical capture probe (V)
[0094] Thymopentin (0.48g, 0.7mmol) was dissolved in 50mL DMSO solution containing compound 7 (0.30g, 0.6mmol); stirred at room temperature and protected from light for 12 hours; after the reaction was completed, the solution was added with frozen ether to obtain Light yellow solid, compound V (38.9% yield).
[0095] 2. Phosphoryl radical capture probe linking thymopentin and dansyl chloride fluorescein (VI)
[0096] Dissolve dansyl chloride fluorescein (0.24g) in 50ml pyridine, add dropwise the free radical trapping probe (V) pyridine solution (0.01mM, 10ml) under stirring at 4℃, then continue stirring for 8h, keep the reactor at 4℃ In the reaction.
[0097] The reaction product was crudely separated by Sephadex LH-20 to remove unre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com