Protein adhered to surface layers of bacteria and application thereof
A protein, application technology, applied in the direction of antibacterial immunoglobulin, bacterial peptide, serum immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Embodiment 1. Identification of Lactobacillus cell surface adhesion protein
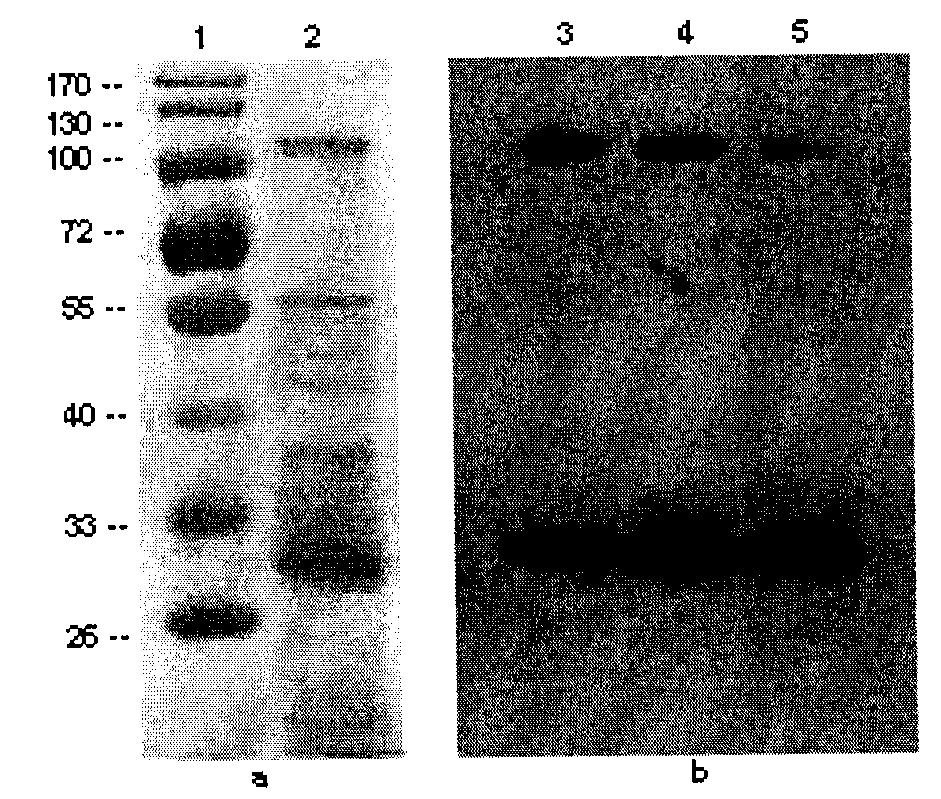
[0130] The surface protein obtained by guanidine hydrochloride combined with ultracentrifugation was subjected to SDS-PAGE electrophoresis and western blotting, and the results showed that the control Marker had multiple bands at positions such as 100-130KD, 55KD, 35-40KD, and 30-33KD ( See figure 2 a). There is a strong positive band at 30KD of the PVDF membrane, and the adhesion protein is located in this band, see figure 2 b.
Embodiment 2
[0131] Example 2. LC-MS / MS and data analysis
[0132] Get the corresponding band at the 30KD place of the PAGE gel for LC-MS / MS and use SEQUEST software (Thermo Fisher Inc.) to carry out database retrieval analysis and ProteinProphet algorithm (Nesvizhskii, A.I., etc., A statistical model for identifying proteins by tandem mass spectrometry.Analytical Chemistry 2003;75:4646-4658) for result filtering. The results showed that the coincidence rate of 5 proteins with the protein library of plant lactic acid bacteria WCFS reached 90%, as shown in Table 2.
[0133] Table 2. Expressed proteins
[0134] Numbering
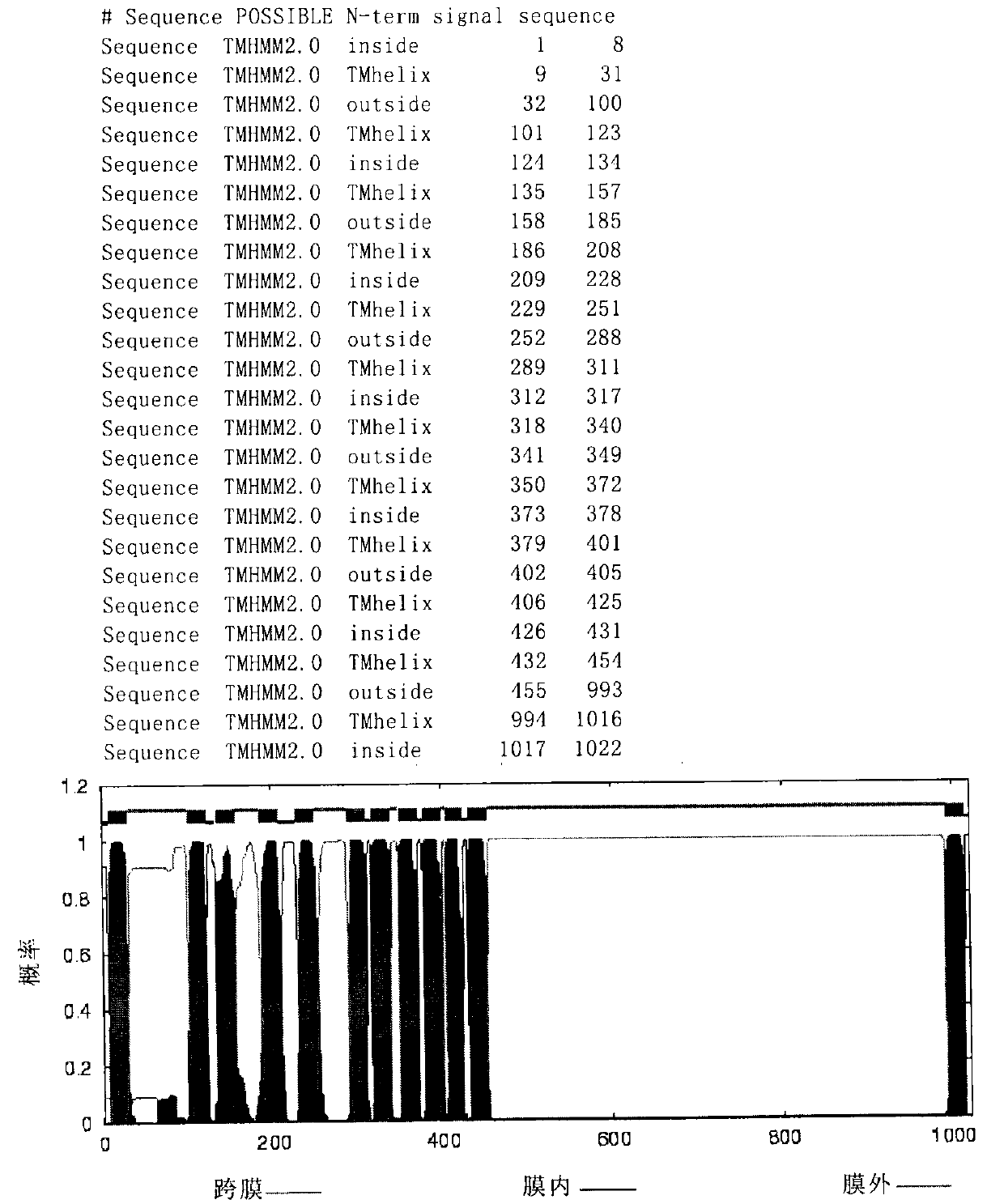
[0135] Among them, the integral membrane protein is divided into three protein fragments, which are respectively called IMP1 (sequence 32-100 in SEQ ID NO: 1), IMP2 (sequence 455-755 in SEQ ID NO: 1), and IMP3 (sequence 455-755 in SEQ ID NO: 1). ID NO: 693-993 sequence in 1).
Embodiment 3
[0136] Example 3. Cloning, expression and purification of proteins
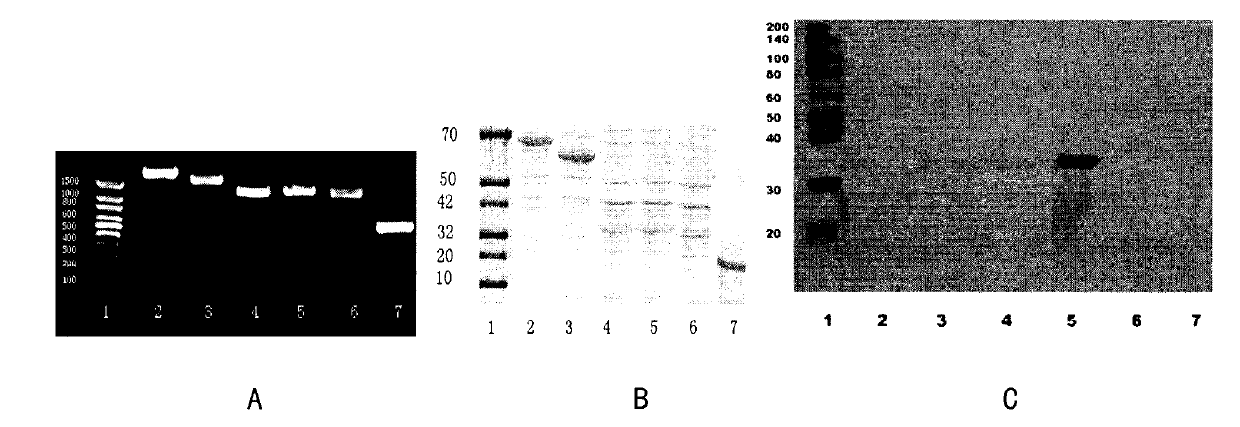
[0137] The target gene of L. plantarum CGMCC No.1258 was amplified by PCR. After electrophoresis, the PCR product showed a single band at the position of 1500bp-500bp in 1% agarose gel, and the result was as expected, see image 3 a.
[0138]The PCR products were separated and purified by agarose gel electrophoresis and recombined into PET-16B vectors. The plasmid was transformed into competent DH10B and sequenced. The results showed that the amplified gene of L. plantarum CGMCC No.1258 was consistent with the data of L plantarum WSFC in Genbank (GenBank accession number: NP_785773.1). Then the plasmid containing the target gene was transfected into E.coli BL21. IPTG induced 3hr. Cells were lysed by ultrasound, and the target protein was extracted and purified with His-tag Fusion Protein Purification Beads. After protein elution, 10% SDS-PAGE electrophoresis separation ( image 3 B) Simultaneously use ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com