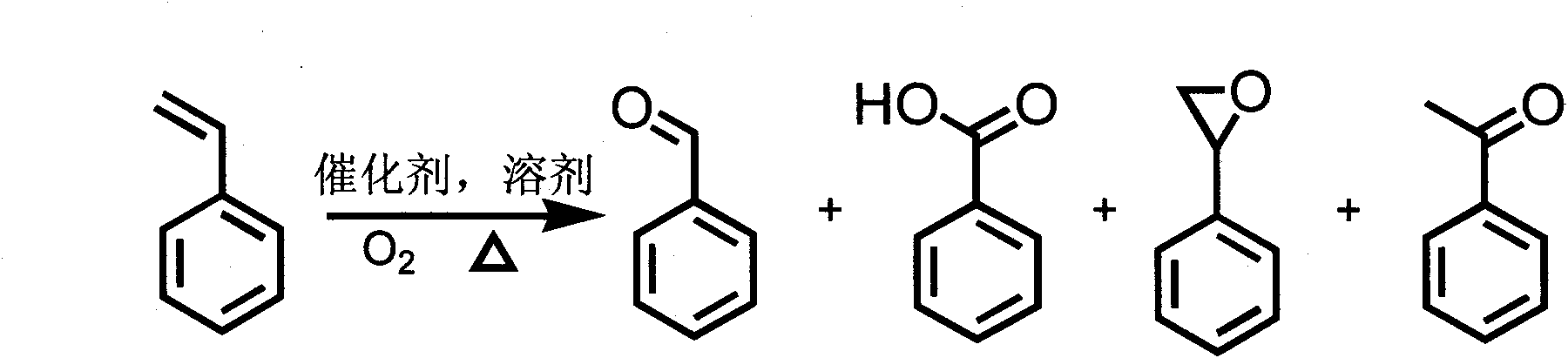
Method for preparing benzaldehyde through catalytic oxidation of styrene
A technology for catalytic oxidation and styrene, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, etc. It can solve the problems that the yield of benzaldehyde is only about 21.18%, it does not conform to green chemistry, and the amount of catalyst is large. , to achieve the effect of high atom utilization, low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
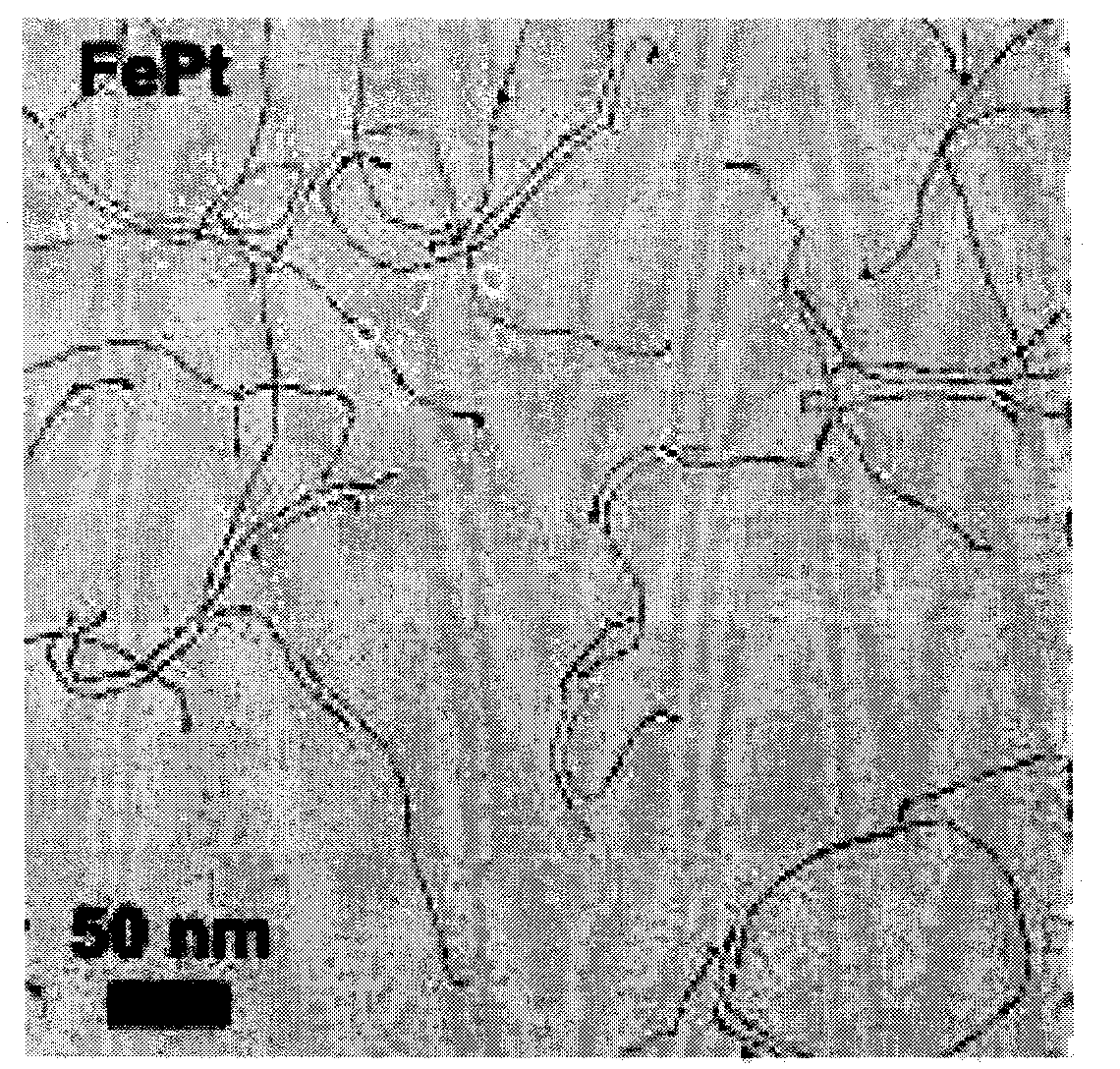
[0023] Synthesis of iron-platinum catalyst: Reduction of platinum acetylacetonate in oleylamine at 160 °C while thermally decomposing iron pentacarbonyl to obtain FePt nanowires with a diameter of 2-3 nm (see Angew.Chem.Int.Ed.2007, 46, 6333-6335) ; Scanning electron microscopy on the obtained nanowires, the results are as follows figure 1 ,From figure 1 It can be seen that the diameter of the iron-platinum nanowires is 2-3 nm.
[0024] In order to facilitate the experimental operation, the iron-platinum nanowires are dispersed in n-hexane, and because the boiling point of n-hexane is low, it is easy to remove, and the dispersion effect on iron-platinum is better, which can prevent the nanowires from agglomerating.
[0025] Under ultrasonic conditions, the above-mentioned iron-platinum nanowires are dispersed in n-hexane, and every 1 m of n-hexane corresponds to 1.6-4 mg of iron-platinum nanowires to obtain an n-hexane solution of iron-platinum nanowires.
Embodiment 2
[0027] 100 μL of n-hexane solution of iron-platinum nanowires was sequentially loaded into the reaction bottle (n-hexane was removed by vacuum pump, leaving catalyst solid); acetonitrile 2 ml; styrene 500 μL; n-dodecane 50 μL (as internal standard). Connect the system with an oxygen bag and a condenser tube, then cool down-vacuumize-release oxygen, cycle 3 to 4 times, put in oxygen, and return to room temperature; the system is heated in an oil bath at 70°C for 24 hours while stirring get the product.
[0028] After the reaction, the mixed solution was centrifuged, and the supernatant was taken for gas-mass chromatography (GC-MS) and gas chromatography (GC) analysis (in the test process, the standard raw material was directly compared to prove that the desired compound was indeed obtained), Result is as follows: the absolute productive rate of benzaldehyde is 38.2%, and benzaldehyde, benzoic acid, the selectivity of benzooxirane are respectively 77.1: 4.1: 18.8, wherein there ...
Embodiment 3
[0030] Put 100 μL of n-hexane iron-platinum nanowire solution in 8 reaction flasks in turn (use a vacuum pump to remove n-hexane and leave the catalyst solid); solvent (see Table 1 below) 2ml; styrene 500 μL; n-dodecane 50 μL (as internal standard). Connect the system with an oxygen bag and a condenser tube, then cool down - vacuumize - release oxygen, cycle 3 to 4 times, put in oxygen, and return to room temperature; then put the 8 reaction systems in an oil bath at 60°C, The product was obtained after heating with stirring for 24 h.
[0031] After the reaction, the mixed solution was centrifuged, and the supernatant was taken for gas-mass chromatography (GC-MS) and gas chromatography (GC) analysis (in the test process, the standard raw material was directly compared to prove that the desired compound was indeed obtained), The results are as follows:
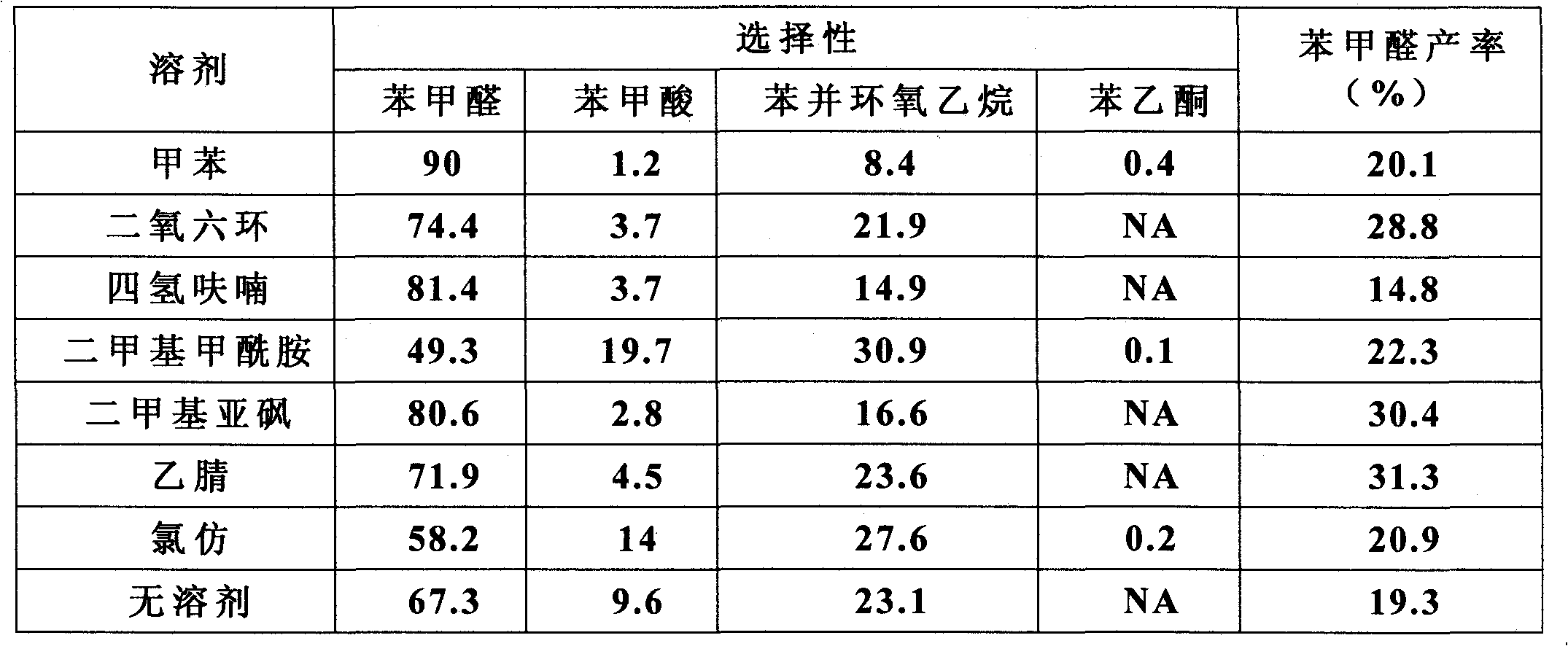
[0032] The impact of different solvents in table 1 on the reaction
[0033]
[0034] As can be seen from the table, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com