Catalyst used for coupling reaction of carbon dioxide and epoxy compound
A technology of epoxy compounds and carbon dioxide, which is applied in the direction of organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, catalyst activation/preparation, etc., can solve the difficulty of separating products from catalysts, high catalytic costs, and technical problems. Complicated and other issues, to achieve the effect of no heavy metal pollution, simple preparation steps, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
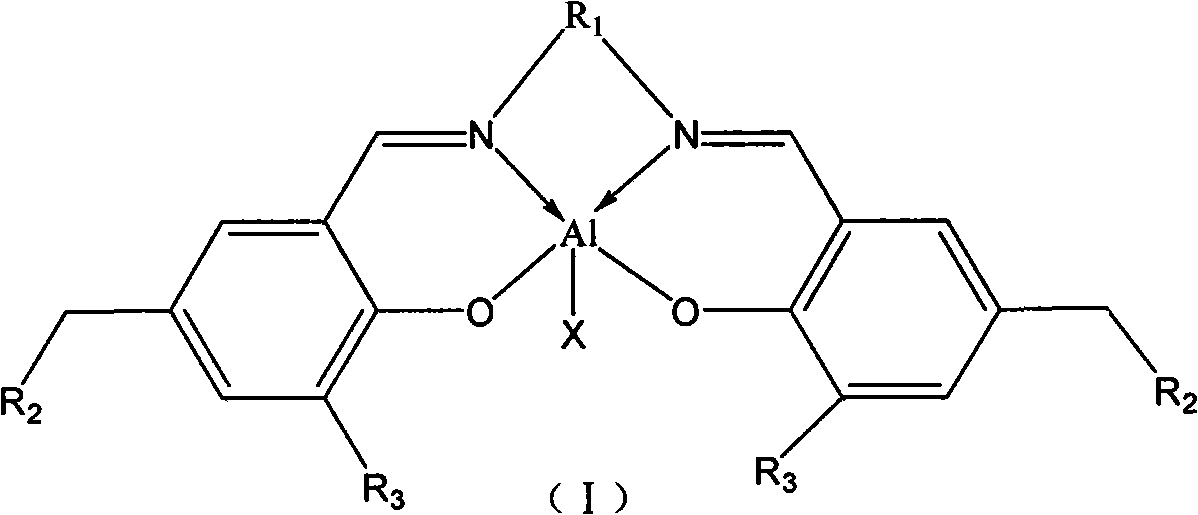
[0033] Embodiment 1: (ligand II 1 synthesis)
[0034] Take a 50mL three-necked flask, pump it three times under an argon atmosphere, and add 0.018mol 3-tert-butyl-5-(methylene-chloro(nitromethylimidazole)) salicylaldehyde (III 1 ), 30ml of ethanol was dissolved, 0.009mol cyclohexanediamine was added to the system, reacted at reflux temperature for 12 hours, stopped the reaction, cooled to room temperature, concentrated under a vacuum of 0.1MPa until the quality of the crude product was constant, and the concentrate was added with 15mL of ethyl acetate A light yellow precipitate appears, filter, collect the filter cake, and drain the filter cake under a vacuum of 0.1MPa to obtain Ligand II 1 , yield 82%.
Embodiment 2
[0035] Embodiment 2: (II 2 )
[0036] With 3-tert-butyl-5-(methylene-chloride (tri-n-butylamine)) salicylaldehyde (III 2) (0.018mol) instead of 3-tert-butyl-5-(methylene-chlorination (nitromethylimidazole)) salicylaldehyde (III 1 ), other operations are with embodiment 1, get II 2 , yield 73%.
Embodiment 3
[0037] Embodiment 3: (II 3 )
[0038] With 3-tert-butyl-5-(methylene-N-imidazole) salicylaldehyde (III 3 ) (0.018mol) instead of 3-tert-butyl-5-(methylene-chlorination (nitromethylimidazole)) salicylaldehyde (III 1 ), other operations are with embodiment 1, get II 3 , yield 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com