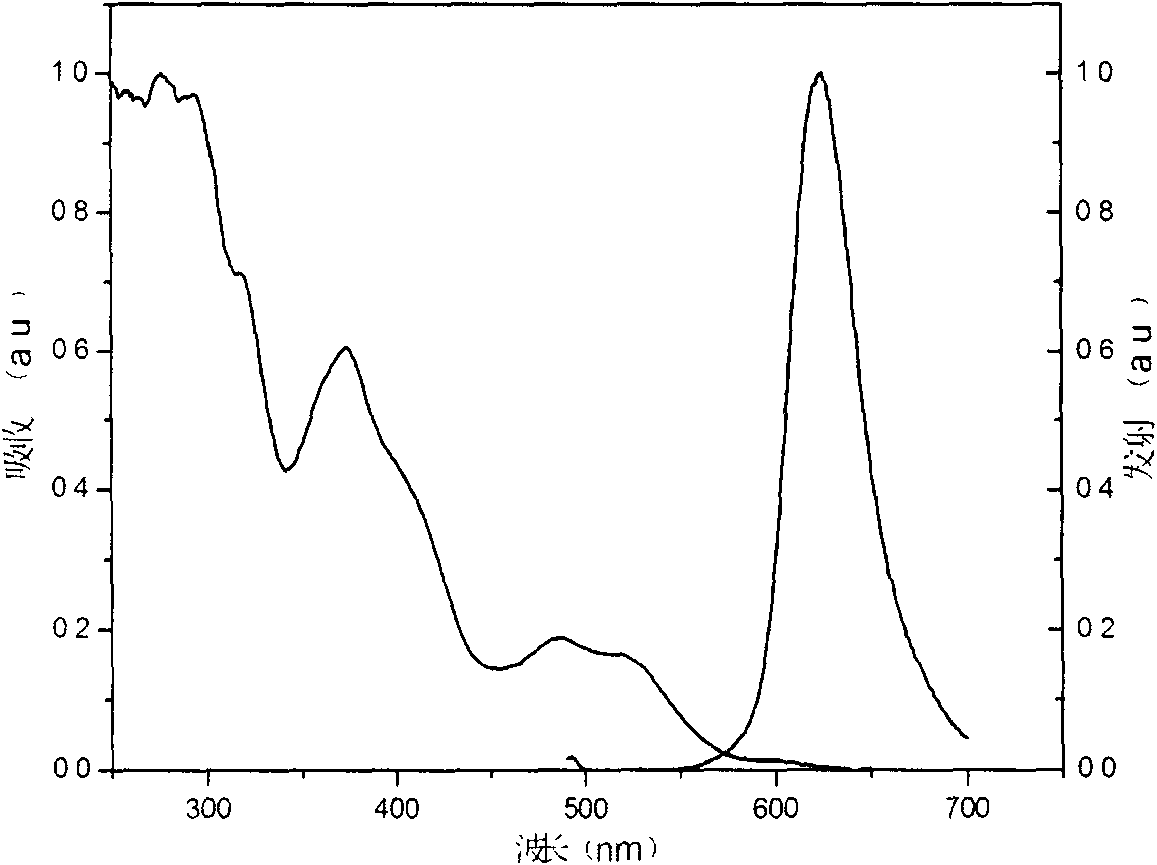
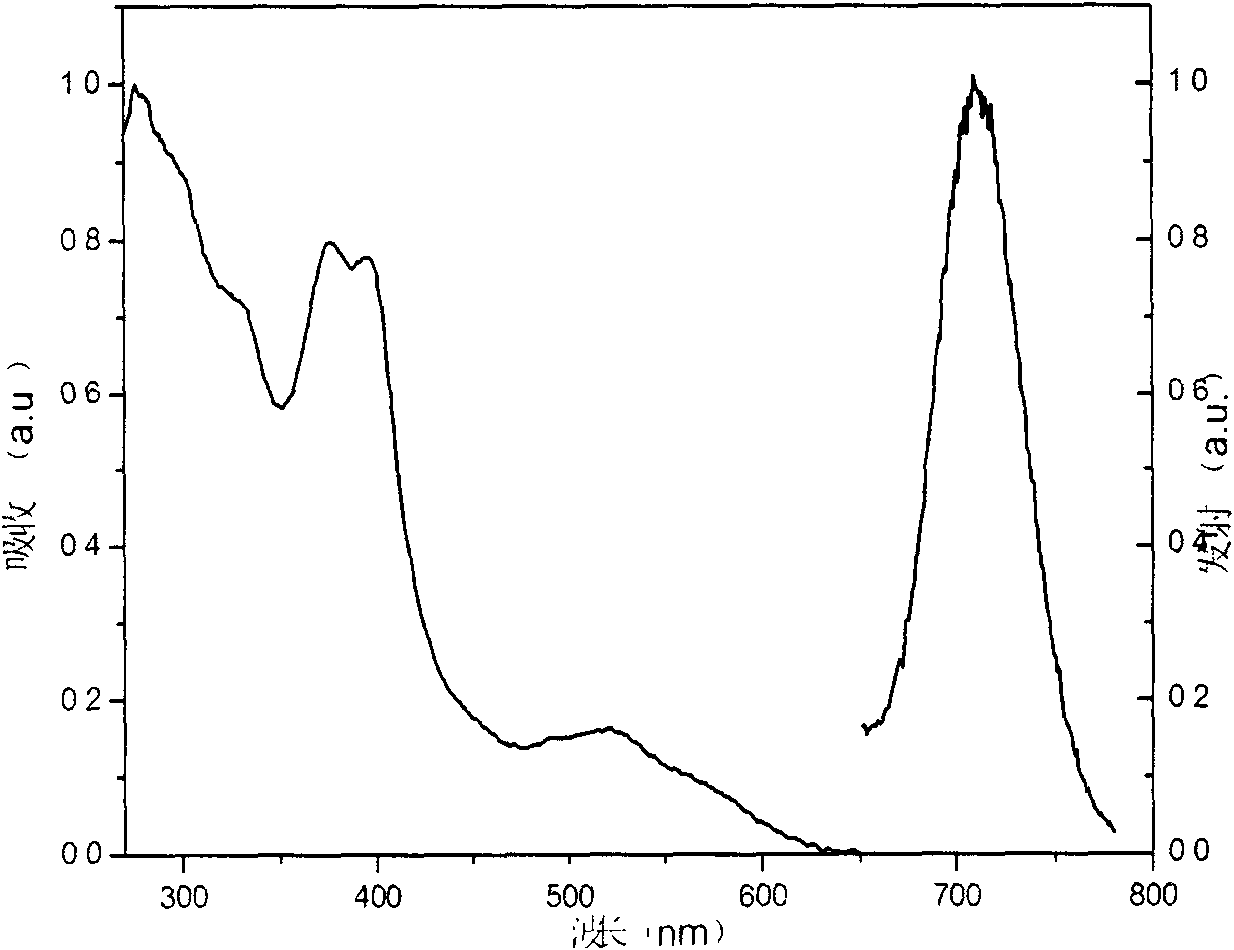
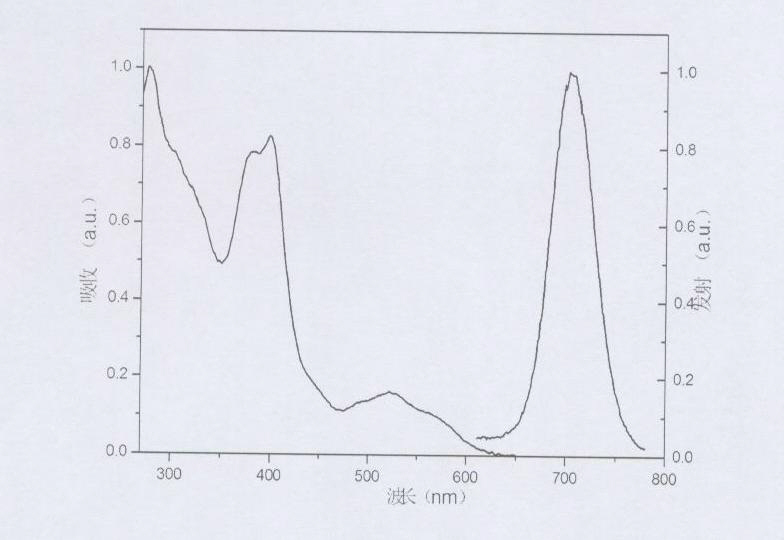
Iridium complex phosphorescent materials with wavelengths from infrared to near-infrared range and preparation method thereof
A technology of iridium complexes and phosphorescent materials, applied in luminescent materials, compounds containing elements of Group 8/9/10/18 of the periodic table, chemical instruments and methods, etc. Agglomeration and other problems, to achieve the effects of low cost, good luminescence performance, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Synthesis of 2-acenaphthylene-5-yl-4-phenyl-quinoline
[0042] Take g aluminum trichloride and 20mL dichloromethane to react for 2h in an ice bath until the aluminum trichloride is completely dissolved, add 1.54g acenaphthylene, and then slowly dropwise dissolve in the dichloromethane solution with mL of acetyl chloride. Pour into a large amount of water, stir overnight, extract with dichloromethane, spin to dry and pass through a column with silica gel (petroleum ether) to obtain a light yellow solid with a yield of 80% 1-acenaphthylene-5-yl-ethanone. Take 0.1g phosphorus pentoxide and 20ml m-cresol into a 100mL single-necked flask and react at 140℃ for 2h, and then take 0.196g 1-acenaphthylene-5-yl-ethanone and 0.197g 2-amino group. In 20ml m-cresol of benzophenone, react at 180°C for 6h. After cooling, it was poured into 300ml of 40% sodium hydroxide solution, extracted with ethyl acetate, spin-dried and passed through the column with silica gel (pure oil et...
Embodiment 2
[0043] Example 2: Synthesis of 6-(4-phenyl-quinolin-2-yl)-benzo[de]isochrome-1,3-dione
[0044] Take 0.357g 2-Acenaphthen-5-yl-4-phenyl-quinoline and 1g potassium dichromate and put them into 10mL acetic acid. After reacting at 120°C for 30 minutes, it was taken out, filtered off with suction, the filter cake was washed with water, the filtrate was extracted with ethyl acetate, and recrystallized with acetic anhydride to obtain the pale yellow product 6-(4-phenyl-quinolin-2-yl)- Benzo[de]isochrome-1,3-dione, the yield is 83%
Embodiment 3
[0045] Example 3: Synthesis of 2-Butyl-6-(4-phenyl-quinolin-2-yl)-benzo[de]isoquinoline-1,3-dione
[0046] Dissolve 5 in 20 mL of ethanol, then add 2 mL of n-butylamine, and then reflux for reaction overnight at 80°C. Then spin off all the solvents and wash off excess n-butylamine with 20 mL of 10% dilute hydrochloric acid. The yellow product 2-butyl-6-(4-phenyl-quinolin-2-yl)-benzo[de]isoquinoline-1,3-dione was obtained with a yield of 95%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com