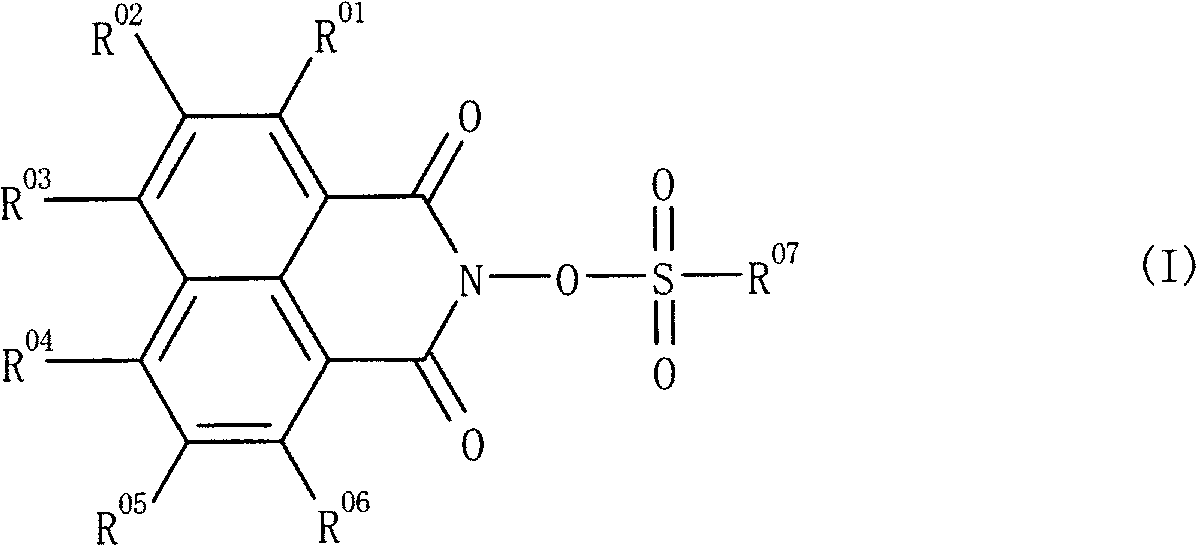
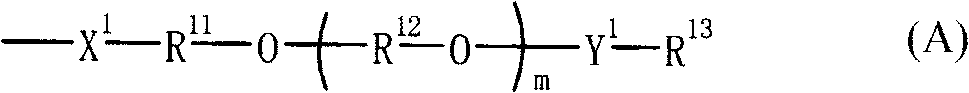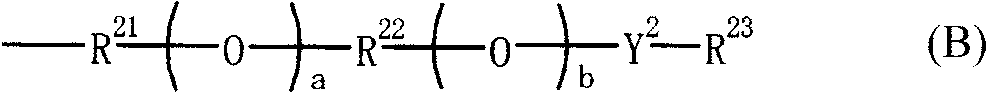Novel sulfonic acid derivative compound and novel naphthalic acid derivative compound
A technology of sulfonic acid derivatives and compounds, which is applied in the field of sulfonic acid derivative compounds, can solve the problems of properties, performance differences, insufficient compatibility and photosensitivity, etc., and achieve good photosensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] [Example 1] Production of Compound A-1
[0134] Add 9.290g (0.0335mol) of 4-bromonaphthalic anhydride, 34.0g dimethyl sulfoxide, 4.140g (0.0369mol) of 1,4-diazabicyclo[2.2.2] into a 100ml four-necked flask Octane was stirred, and the inside of the system was set as a nitrogen atmosphere. 3.170 g (0.0352 mol) of butanethiol was added dropwise below 30°C, and stirred at 40°C for 5 hours. 70 ml of methanol was added to the reaction liquid, ice-cooled, stirred for 30 minutes, and the precipitated crystals were collected by filtration. This was dried to obtain yellow crystals of the target compound A-1. Yield 7.15 g (yield 74.6%), based on HPLC (column: Inertsil ODS-24.6 mm × 250 mm manufactured by GL Sciences Inc., solvent: acetonitrile / water = 7 / 3, L-7455 diode array manufactured by Hitachi, Ltd. The purity of detector detection wavelength (230nm) is 97.4%. Based on deuterated dimethyl sulfoxide solvent 1 The measurement results of H-NMR are shown in Table 1-1.
Embodiment 2
[0135] [Example 2] Production of compound I-1
[0136] 6.87 g (0.0240 mol) of compound A-1, 27.5 g of dimethylformamide, and 2.00 g (0.0288 mol) of hydroxylamine hydrochloride were added to a 100 ml four-necked flask, stirred, and a nitrogen atmosphere was set in the system. 2.40 g (0.0288 mol) of 48 weight% sodium hydroxide aqueous solution was dripped at 30 degreeC or less, and it stirred at room temperature for 2 hours. 27.5 ml of ion-exchanged water was added to the reaction liquid, ice-cooled, stirred for 30 minutes, 1.00 g (0.0096 mol) of 35% by weight aqueous hydrochloric acid was added, and stirred for 1 hour. The precipitated crystals were collected by filtration and dried to obtain yellow crystals of the target compound I-1. Yield 7.04 g (yield 97.4%), based on HPLC (column: Inertsil ODS-24.6 mm × 250 mm manufactured by GL Sciences Inc., solvent: acetonitrile / water = 7 / 3, L-7455 diode array manufactured by Hitachi, Ltd. The purity of the detector detection waveleng...
Embodiment 3
[0137] [Example 3] Production of Compound S-28
[0138]Add 3.01g (0.0100mol) of compound I-1, 17.7g of dichloroethane, and 1.26g (0.0125mol) of triethylamine into a 50ml four-necked flask, stir and dissolve, then set the system as a nitrogen atmosphere . 5.77 g (0.0110 mol) of 48 mass % d-camphorsulfonyl chloride dichloroethane solutions were dripped at 20 degreeC, and it stirred for 2 hours. 20 ml of ion-exchanged water and 10 ml of dichloromethane were added to the reaction solution and stirred, and the organic layer obtained by oil-water separation was washed twice with 20 ml of 0.5% by weight aqueous sodium hydroxide solution and 20 ml of 3% by weight aqueous hydrochloric acid solution. After washing once and four times with 30 ml of ion-exchanged water, the solvent was distilled off from the dichloromethane layer to obtain the target product as a yellow solid. Yield 4.696g (yield 90.9%), based on HPLC (column: CAPCELL PAK C8 DD 4.6mm×250mm manufactured by Shiseido, solv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com