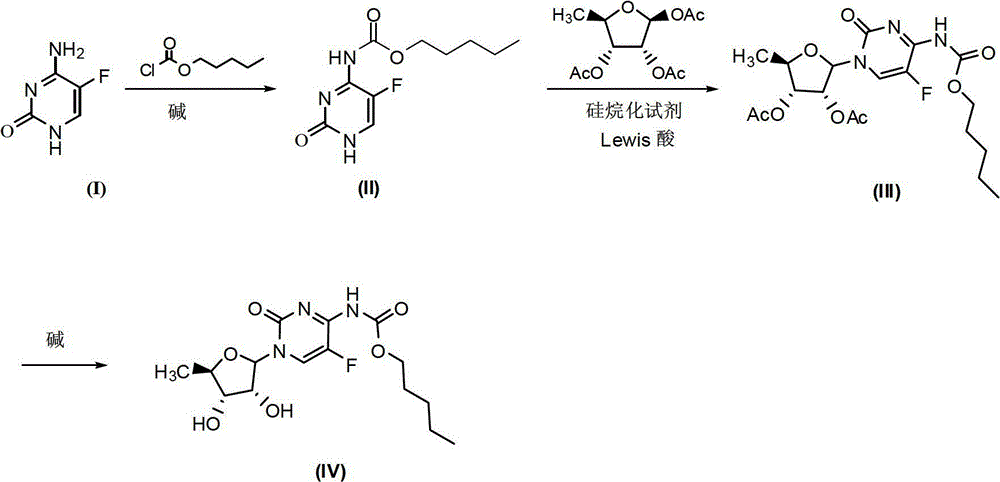
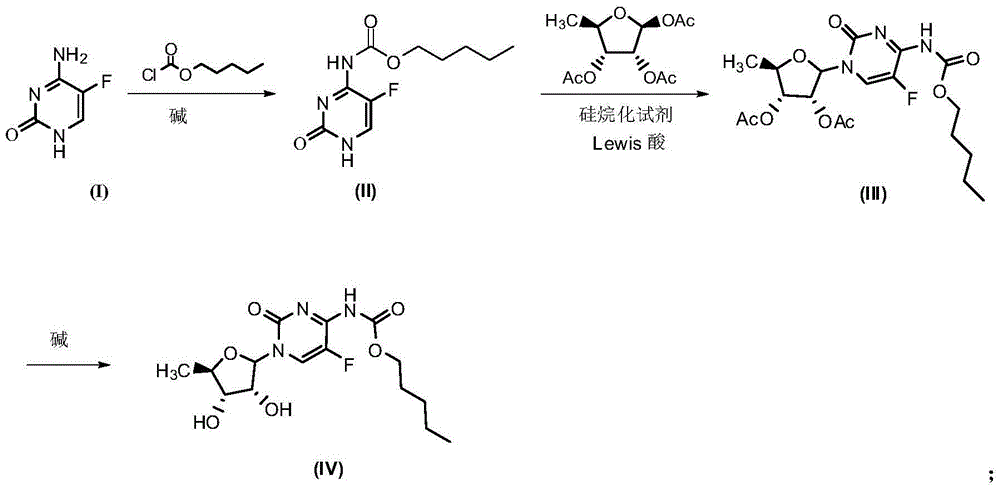
Synthesis method of capecitabine
A capecitabine and synthesis method technology, applied in the field of synthesis of small molecule chemical drug capecitabine, can solve the problems of high production cost, difficult to evaporate, highly toxic phosgene, etc., achieve mild reaction conditions, reduce treatment Loss, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1.1 Synthesis of compound of formula (II) (method 1)
[0025] Dissolve 10g (0.08mol) of the compound of formula (I) 5-fluorocytosine in 60ml of pyridine, and slowly add 13.5ml (0.096mol) of n-pentyl chloroformate to the resulting solution in an ice bath, and the addition is complete Then transfer to 110°C oil bath for 6h reaction. After the reaction was completed, ice water was added, and 18.5 g of white solid, namely the compound of formula (II), was obtained by filtration, with a yield of 98.4%.
[0026] 1 H-NMR (DMSO-d 6 )δ: 11.13 (brs, 2H, -CONH); 7.96 (s, 1H, Ar-H); 4.05 (t, 2H, J=6.8, -CH 2 O); 1.62-1.56 (m, 2H, -CH 2 ); 1.31-1.29 (m, 4H, -CH 2 -CH 2 -); 0.89-0.85(t, 3H, -CH 3 ).
[0027] 1.2 Synthesis of compound of formula (II) (method 2)
[0028] Dissolve 10g (0.08mol) of the compound of formula (I) 5-fluorocytosine in 50ml of DMSO, and add 28.5ml (0.16mol) of diisopropylethylamine under stirring, and slowly add chloroformic acid dropwise at room te...
Embodiment 2
[0031] 2.1 Synthesis of compound of formula (III) (method 1)
[0032] Dissolve 18.5g (0.076mol) of the compound of formula (II) in 100ml of anhydrous dichloromethane, and add 20ml (0.08mol) of BSA dropwise to the resulting solution, and a mixed solution is obtained after the addition; Boron trifluoride-ether 10.6g (0.075mol) of the complex was dissolved in 150ml of anhydrous dichloromethane, and 20g (0.077mol) of 1,2,3-O-acetyl-5-deoxy-D-ribofuranose was added and stirred, and the stirred solution Add to the above mixed solution, and stir the reaction for 7 hours in an oil bath at 30°C under nitrogen protection. After the reaction, wash with saturated sodium bicarbonate (150×3ml), saturated sodium chloride (150×3ml), anhydrous sodium sulfate dry. After spin-drying, 30 g of a yellow semi-solid product, namely the compound of formula (III), was obtained, with a yield of 91%. Wherein, the product does not need to be purified and can be directly used in the next reaction.
[...
Embodiment 3
[0039] Synthesis of the compound of formula (IV)
[0040] Dissolve 30g of the compound of formula (III) in 60ml of methanol, and slowly add 165ml of 0.5mol / L lithium hydroxide solution dropwise to the resulting solution under ice-salt bath conditions, and then continue the reaction under this condition for 0.5h, and the reaction ends Then adjust the pH to about 5-6 with 1M hydrochloric acid, extract with dichloromethane (150ml), wash with saturated sodium bicarbonate (150×3ml), wash with saturated sodium chloride (150×3ml), and dry over anhydrous sodium sulfate. 21.8 g of a white solid, namely the compound of formula (IV), was obtained with a yield of 90%.
[0041] 1 H-NMR (DMSO-d6) δ: 10.53 (brs, 1H, -CONH); 8.00 (s, 1H, -ArH); 5.67-5.66 (m, 1H, 1'-H); 5.43-5.42 (m, 1H, 2'-H); 5.07-5.05(m, 1H, 4'-H); 4.04-4.02(m, 3H, -CH 2 O, 3'-H); 3.89-3.86(m, 1H, 2'-OH); 3.70-3.67(m, 1H, 3'-OH); 1.61-1.58(m, 2H, -CH 2 ); 1.32-1.29 (m, 4H, -CH 2 -CH 2 ); 1.24(s, 3H, 4'-CH 3 ); 0.88...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com