Antibacterial peptide LZ1 and application of antibacterial peptide in preparation of antibacterial medicament
An antibacterial peptide and antibacterial drug technology, applied in the field of antibacterial drug preparation, can solve the problems of low antibacterial activity, cytotoxicity of host cells, etc., and achieve the effects of strong bactericidal effect, extremely low eukaryotic cytotoxicity, and convenient artificial synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the preparation of antimicrobial peptide LZ1
[0021] 1. Chemical synthesis method of antimicrobial peptide LZ1: According to the amino acid sequence described in the summary of the invention, its entire sequence was synthesized with an automatic peptide synthesizer (433A, Applied Biosystems), and purified by HPLC reverse-phase column chromatography desalting.
[0022] Ⅱ. Molecular weight was determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF).
[0023] Ⅲ. The purity of the purified antibacterial peptide LZ1 was identified by high performance liquid chromatography (HPLC), the molecular weight was determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF), the isoelectric point was determined by isoelectric focusing electrophoresis, and an automatic amino acid sequencer was used to determine Amino acid sequence structure.
[0024] The antibacterial peptide LZ1 co...
Embodiment 2
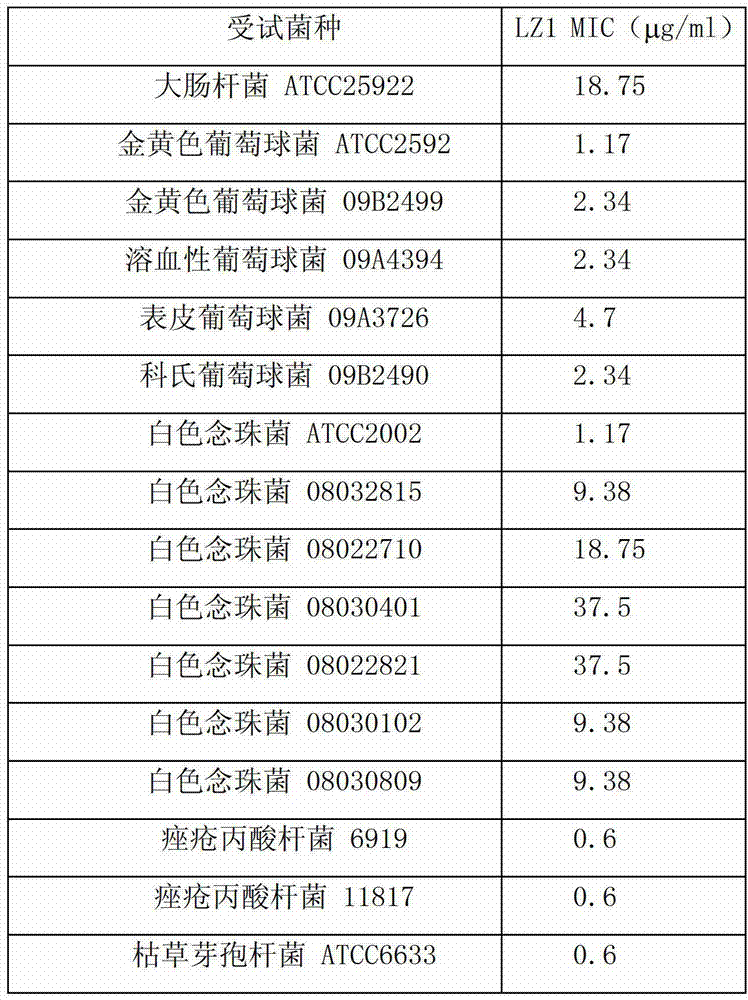
[0025] Embodiment 2: antibacterial experiment of antimicrobial peptide LZ1:
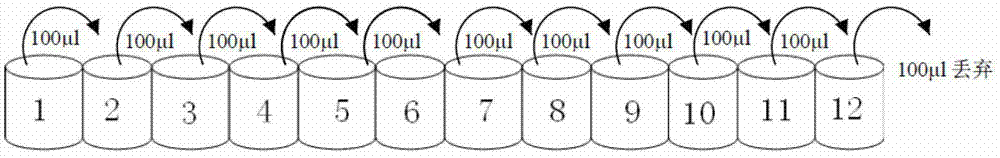
[0026] Minimum inhibitory concentration (minimal inhibitory concentration, MIC): is the lowest sample concentration where no bacterial growth can be detected. Using the two-fold dilution method, such as figure 1 , the specific method is as follows:
[0027] Bacteria were inoculated on Luria-Bertani (LB) solid medium and cultured upside down in a 37°C incubator. After the colony grows, pick a single colony with an inoculation loop and transfer it to LB liquid medium, and shake it in a 37°C incubator until the logarithmic growth phase. Detect the OD600 of the bacterial solution on the ultraviolet spectrophotometer, according to 1OD600=1×10 9 CFU / ml Dilute the bacterial solution to 2×10 with liquid LB medium 5 CFU / ml. Add 100 μl of LB liquid medium to each well of a sterile 96-well plate, then add 100 μl of antimicrobial peptide samples diluted to a certain concentration to the first well and filte...
Embodiment 3
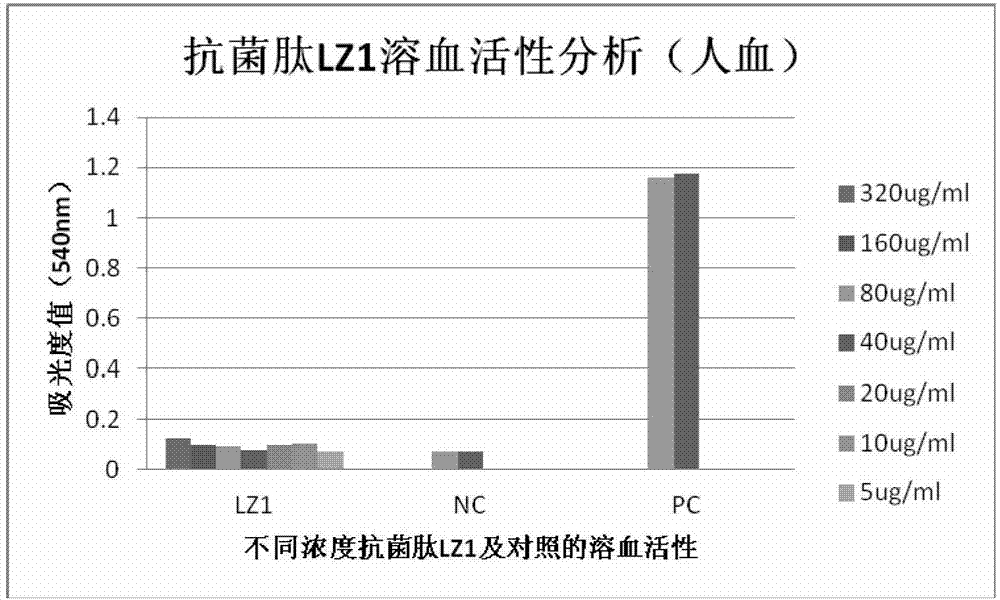
[0032] Example 3: Hemolytic Activity Experiment of Antimicrobial Peptide LZ1:
[0033] Rabbit heart blood collection or human vein blood collection, the collected blood and Alsever Solution (Alsever Solution, 8.0g sodium citrate, 0.55g citric acid, 20.5g glucose, 4.2g NaCl, add deionized water to 1L, adjust the pH to 6.1 , stored at 4°C after autoclaving) were mixed at a ratio of 1:1 and placed in a centrifuge tube, centrifuged at 1000 rpm for 5 minutes, and washed with normal saline until the supernatant was no longer red. Dilute the washed red blood cells with normal saline to 10 7 -10 8 concentrated suspension. The above-mentioned diluted erythrocyte suspension was incubated with samples of different concentrations dissolved in normal saline at 37°C for 30 minutes, then centrifuged at 1000 rpm for 5 minutes, and the supernatant was measured for absorbance at 540 nm. Normal saline was used as the negative control, and Triton X-100 was used as the positive control. The he...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com