Iminazole type ionic liquid reversed phase electrochromatography organic monolithic column
An ionic liquid, imidazole type technology, applied in the field of analytical chemistry, to achieve the effect of good shape, low rigidity improvement and irreversible tailing phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Pretreatment of the column
[0022] The quartz capillary empty column is first flushed with 0.1 mol / L NaOH for 2 h to 3 h, then with deionized water for 10 min, then with 0.1 mol / L HCl solution for about 1 h, and then with methanol for 30 min. Blow dry and set aside.
[0023] (2) This step is optional.
[0024] Add a 1:1 mixture of methanol and methacryloxypropyltrimethoxysilane to the capillary treated in step 1, and react at 60°C for 12 to 24 h. Then rinse with methanol for 20 min to 30 min. Blow dry with nitrogen at 70°C.
[0025] (3) In-column synthesis
[0026] Lauryl methacrylate (functional monomer), ethylene dimethacrylate (crosslinking agent), 1-allyl-3-methylimidazole hexafluorophosphate, porogen and initiator Prepared according to the following mass percentage data:
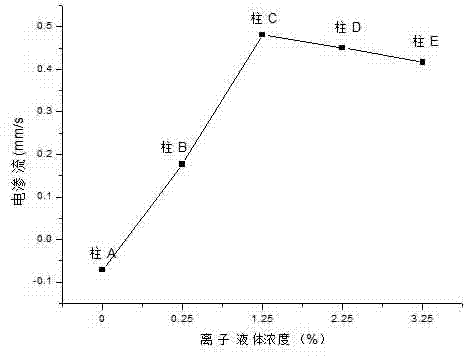
[0027] Column A: Neutral compound 25%, methanol: 2.5%, n-propanol: 25%, 1,4-butanediol: 36.5%, initiator azobisisobutyronitrile 1%.
[0028] Column B: Neutral compound 24.75%, 1-allyl-3-methylimidaz...
Embodiment 2
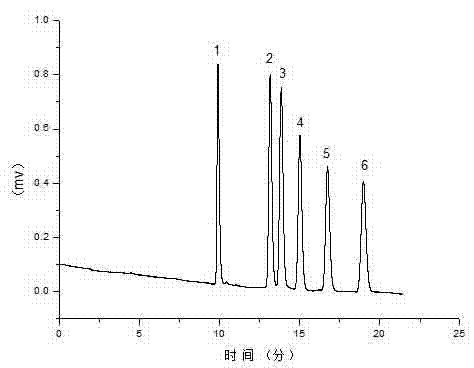
[0037] In order to verify the separation performance of the developed imidazole-type ionic liquid reversed-phase electrochromatographic organic monolithic column for neutral homologues, the monolithic column D prepared above was used with acetonitrile: phosphate buffer salt (5 mmol / L, pH=7.0) = 70:30 is the mobile phase, the separation voltage is +10 kV, the auxiliary pressure is 1.2 MPa, and the pump flow rate is 0.1 ml / min. Neutral benzene series are baseline separated on the positively charged hydrophobic interaction organic monolithic column. The elution peaks are: 1 thiourea, 2 toluene, 3 ethylbenzene, 4 propylbenzene, 5 butylbenzene, 6 pentylbenzene.
Embodiment 3
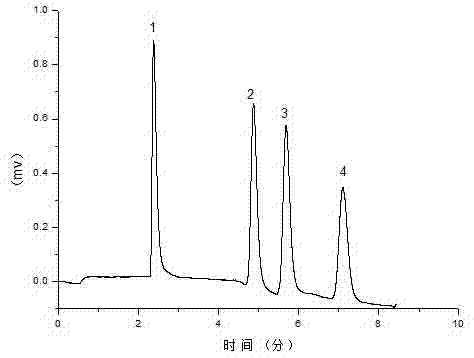
[0039] In order to verify the separation performance of the developed imidazole-type ionic liquid reversed-phase electrochromatographic organic monolithic column for polycyclic aromatic hydrocarbons, the monolithic column D prepared above was used with acetonitrile: phosphate buffer salt (5 mmol / L, pH=7.0)=80 : 20 is the mobile phase, the separation voltage is +15 kV, the auxiliary pressure is 1.2 MPa, and the pump flow rate is 0.1 ml / min. Polycyclic aromatic hydrocarbons achieve baseline separation on the reversed-phase electrochromatographic organic monolithic column and the elution peak The order is: 1 thiourea, 2 naphthalene, 3 phenanthrene, 4 anthracene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com