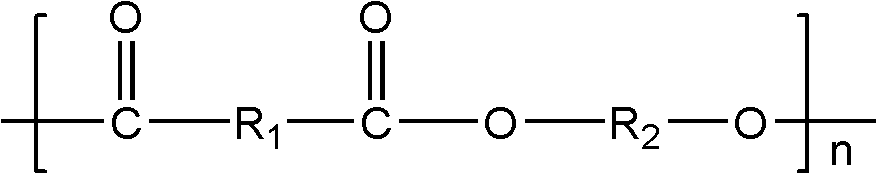
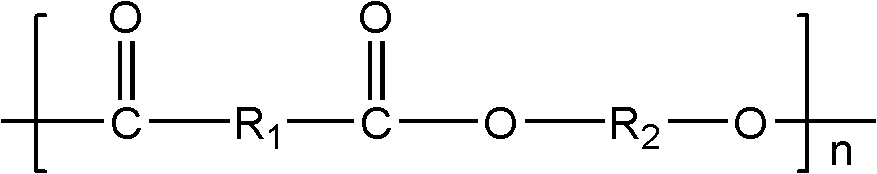
Biodegradable aromatic-aliphatic copolyester and preparation method thereof
An aliphatic copolyester and biodegradation technology, applied in the field of biodegradation of aromatic-aliphatic copolyester and its preparation, can solve the problem of weakening the advantages of aromatic-aliphatic copolyester and affecting the biodegradation of copolyester materials Performance, the content of aromatic dibasic acid should not be too high, etc., to achieve the effect of increasing irregularity, improving hydrophilicity and dyeing performance, and meeting the requirements of diversity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
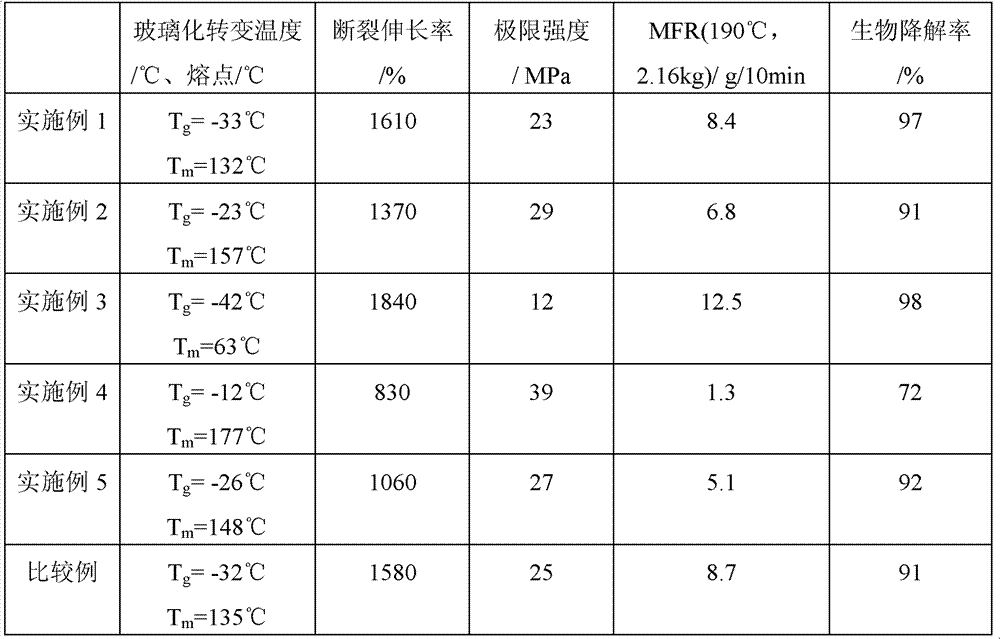
[0054] Add 73g of adipic acid, 75g of terephthalic acid, 13g of isophthalic acid-5-sodium sulfonate, 220g of 1,4-butanediol, and 0.10g of tetra-n-butyl titanate into a tank equipped with a mechanical stirring device In a 500mL three-neck bottle. After all the materials have been added, equip the flask with a condensation device, vacuumize and fill with nitrogen three times to remove the oxygen in the reaction vessel to ensure that the esterification reaction is carried out under nitrogen conditions. Heating to 225°C. After the reactants form a homogeneous system, control the reaction temperature so that the esterification reaction is stirred at 225°C for 3 hours. During this process, water is distilled from the reaction mixture as a by-product until the amount of the distillate reaches 92% of the theoretical calculation. (The theoretically calculated amount of water is twice the sum of the molar amounts of adipic acid, terephthalic acid and isophthalic acid-5-sodium sulfonate...
Embodiment 2
[0058] Add 70g dimethyl adipate, 107g dimethyl terephthalate, 15g dimethyl isophthalate-5-sodium sulfonate, 230g 1,4-butanediol, 0.15g zinc acetate to the container In a 500mL three-necked bottle with a mechanical stirring device. After all the materials have been added, equip the flask with a condensation device, vacuumize and fill with nitrogen three times to remove the oxygen in the reaction vessel to ensure that the transesterification reaction is carried out under nitrogen conditions. Heat to 220°C. After the reactants form a homogeneous system, control the reaction temperature so that the transesterification reaction is stirred at 220°C for 3.5 hours. During this process, methanol is distilled from the reaction mixture as a by-product until the amount of distillate It reaches 92% of the theoretical calculation amount. (The theoretically calculated amount of methanol is twice the sum of the molar amounts of dimethyl adipate, dimethyl terephthalate and dimethyl isophthala...
Embodiment 3
[0062] 41g succinic acid, 51g adipic acid, 47g terephthalic acid, 5g isophthalic acid-5-sodium sulfonate, 93g ethylene glycol, 135g 1,4-butanediol, 0.15g zinc acetate, 0.05 g tetraisooctyl titanate was added to a 500mL three-necked flask equipped with a mechanical stirring device. After all the materials have been added, equip the flask with a condensation device, vacuumize and fill with nitrogen three times to remove the oxygen in the reaction vessel to ensure that the esterification reaction is carried out under nitrogen conditions. Heating to 230°C. After the reactants form a homogeneous system, control the reaction temperature so that the esterification reaction is stirred at 230°C for 2 hours. During this process, water is distilled from the reaction mixture as a by-product until the amount of the distillate reaches 92% of the theoretical calculation. (The theoretically calculated amount of water is twice the sum of the molar amounts of succinic acid, adipic acid, tereph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com