Fulvestrant phosphate derivative and preparation method and application thereof
A technology of fulvestrant phosphate and fulvestrant, which is applied in the direction of drug combinations, pharmaceutical formulas, steroids, etc., can solve the problems of poor stability of fulvestrant raw materials, achieve simple and fast purification methods, and synthesize Convenience and clear structural features
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A fulvestrant cyclophosphate derivative of the following general formula was prepared:
[0023]
[0024] 1. Preparation of 3-O-[4-(3-chlorophenyl)-2-oxo-1,3,2-dioxaphosphacyclohexyl]fulvestrant (F01)
[0025] Add fulvestrant (0.2g, 0.33mmol) to a 50ml two-necked bottle, dissolve with anhydrous tetrahydrofuran (10ml), under nitrogen protection, add a solution of N,N-lithium diisopropylamine in tetrahydrofuran (0.34ml, 0.68 mmol), the reaction was stirred at room temperature for 2.5 h, and 4-(3-chlorophenyl)-2-(4-nitrophenoxy)-2-oxo-1,3,2-dioxaphosphine was added alkane (0.38 g, 1.0 mmol), and the reaction was stirred for 48 h under nitrogen protection. Adjust the pH to 6-7 with 1M dilute hydrochloric acid, concentrate to remove tetrahydrofuran, add dichloromethane (20ml) to dissolve, wash three times with distilled water and saturated brine successively, and perform silica gel column chromatography (petroleum ether:ethyl acetate=1:3) It was purified and concentrated...
Embodiment 2
[0062] The fulvestrant diethyl phosphate derivatives shown below were prepared.
[0063]
[0064] Add fulvestrant (0.5g, 0.8mmol) to a 100ml three-necked flask, fill with nitrogen protection, add dried dichloromethane (40ml), magnetically stir to dissolve it completely, cool with an ice-salt bath to maintain the temperature of the system At -10°C, phosphorus oxychloride (0.15ml, 1.6mmol) was added dropwise to the reaction system with a syringe, the ice bath was removed after 1 hour, the temperature was raised to 20°C, absolute ethanol (0.5ml, 8mmol) was added, and the reaction was stirred. After 2 hours, after TLC detected the reaction, the solvent was evaporated under reduced pressure, dissolved in dichloromethane, washed with water, dried with anhydrous magnesium sulfate, and subjected to silica gel column chromatography to obtain 0.2 g of the target compound with a yield of 33%.
[0065] 1 H-NMR (CDCl 3 , 500MHz, characteristic hydrogen) δ7.22 (d, J=8.5Hz, 1H), 6.95 (d...
Embodiment 3
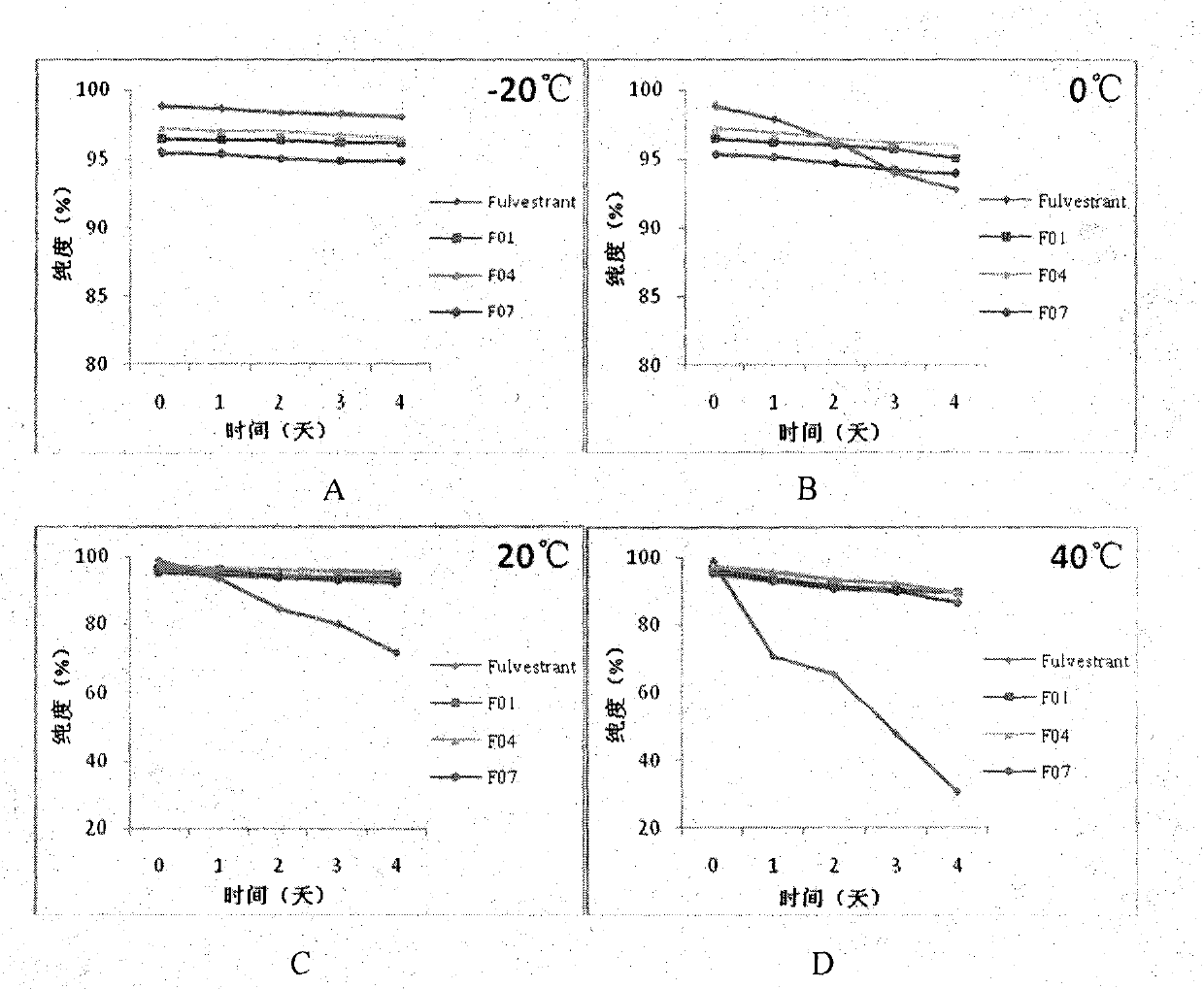
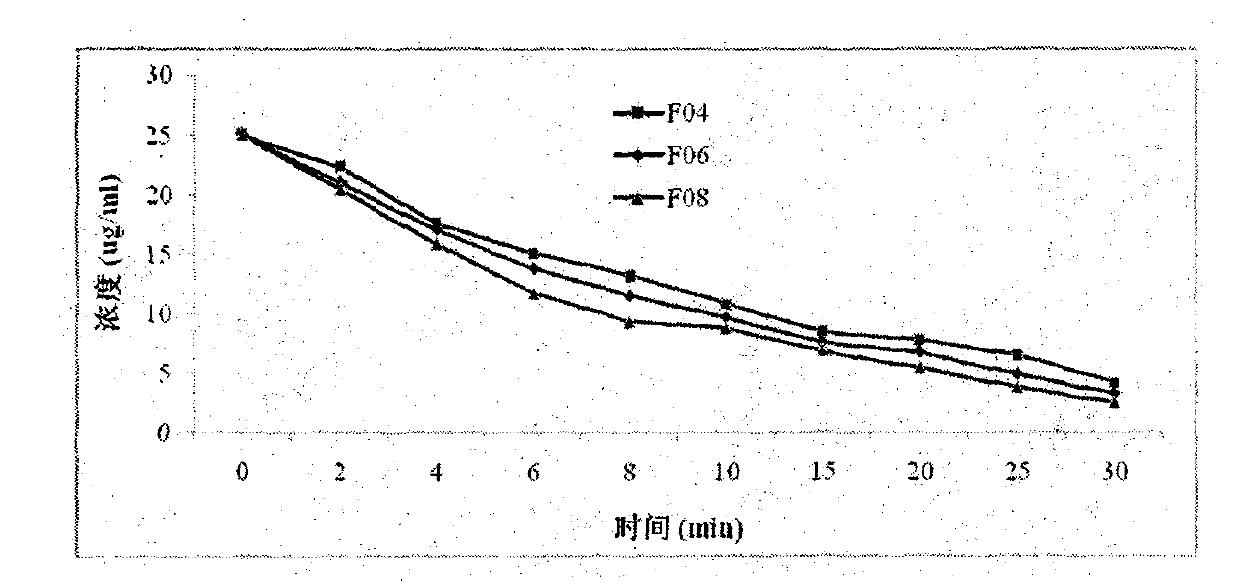
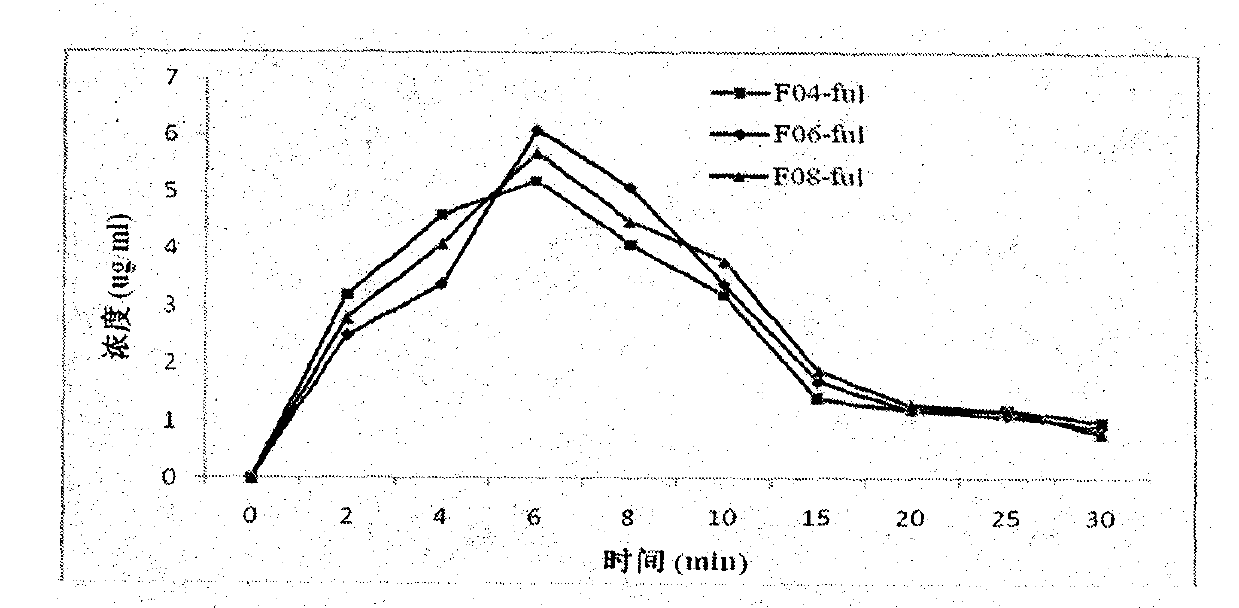
[0067] In vitro stability test of fulvestrant cyclophosphate derivatives of the present invention
[0068] The inventors have proved that the fulvestrant cyclic phosphate derivative of the present invention has better stability than the original drug through stability experiments at different temperatures and different times.
[0069] The stability test method of compound F01 of the present invention, F04, F07 and original drug Fulvestrant is as follows:
[0070] The target compound and fulvestrant were stored at -20°C, 0°C, 20°C, and 40°C for 1-4 days. After methanol was dissolved, the purity of the compound was detected by high performance liquid chromatography. The conditions are as follows:
[0071] Instrument model: Agilent1100 high performance liquid chromatograph
[0072] Mobile phase: 85% methanol: 15% water;
[0073] UV detection wavelength: 210nm
[0074] Injection volume: 10μL
[0075] The experimental results are as figure 1 As shown, the results show that th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com