Ketoprofen solution and preparation method thereof
A technology of ketoprofen and solution, which is applied in the fields of medical formulation, drug delivery, bone diseases, etc., can solve problems such as rupture and rancidity, complicated preparation process, and increased environmental pollution, and achieves increased routes of administration, simple preparation process, and clinical The effect of application security
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
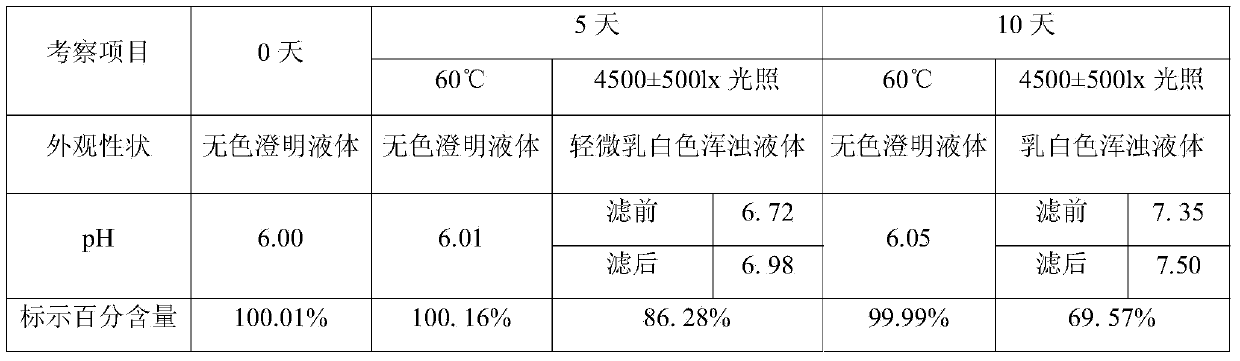
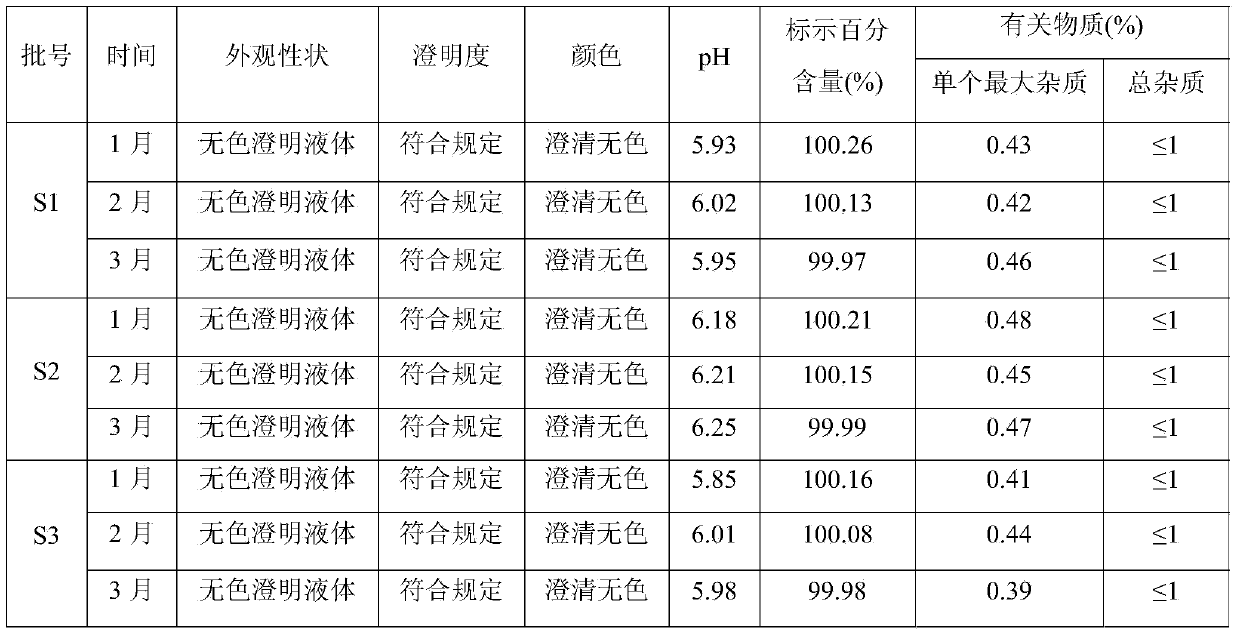
[0020] Embodiment 1, the preparation of ketoprofen solution
[0021] Prescription: ketoprofen 0.01g, sodium acetate 0.1g, appropriate amount of glacial acetic acid, water for injection added to 100mL.
[0022] Preparation method: take 50 mL of water for injection, add the prescribed amount of sodium acetate, stir and mix, then add the prescribed amount of ketoprofen, stir at 50 ± 5 ℃ until completely dissolved, after the liquid is cooled to room temperature, add glacial acetic acid Adjust the pH value to 6.0, dilute the volume to 100 mL with water for injection, mix well, and filter.
Embodiment 2
[0023] Embodiment 2, the preparation of ketoprofen solution
[0024] Prescription: ketoprofen 0.01g, sodium acetate 0.2g, appropriate amount of glacial acetic acid, add water for injection to 100mL.
[0025] Preparation method: take 50 mL of water for injection, add the prescribed amount of sodium acetate, stir and mix, then add the prescribed amount of ketoprofen, stir at 50 ± 5 ℃ until completely dissolved, after the liquid is cooled to room temperature, add glacial acetic acid Adjust the pH value to 6.0, dilute the volume to 100 mL with water for injection, mix well, and filter.
Embodiment 3
[0026] Embodiment 3, the preparation of ketoprofen solution
[0027] Prescription: ketoprofen 0.1g, sodium acetate 1g, appropriate amount of glacial acetic acid, water for injection added to 100mL.
[0028] Preparation method: take 50 mL of water for injection, add the prescribed amount of sodium acetate, stir and mix, then add the prescribed amount of ketoprofen, stir at 50 ± 5 ℃ until completely dissolved, after the liquid is cooled to room temperature, add glacial acetic acid Adjust the pH value to 6.0, dilute the volume to 100 mL with water for injection, mix well, and filter.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com