Preparation method and application of artificial antimicrobial peptide MA-D4
An antimicrobial peptide and artificial technology, applied in the field of genetic engineering, can solve the problems of low biological activity and hemolytic activity of natural antimicrobial peptides, and achieve the effect of strong broad-spectrum antibacterial activity, good stability and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
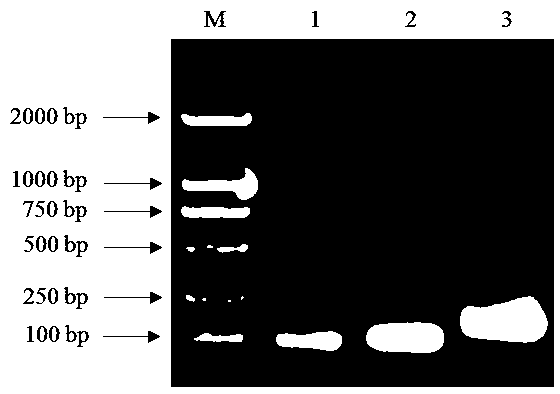
[0022] Example 1: Construction of prokaryotic expression vectors and genetically engineered bacteria.
[0023] According to the amino acid sequences of the mature peptides of the antimicrobial peptide Magainin (No. 474452717) and Dermaseptin-4 (No. P84924.1) in GenBank, the methionine (Met) at position 21 of the antimicrobial peptide Magainin was replaced with tryptophan (Trp) , artificially designed the amino acid sequence SEQ ID No.1 of the artificial antimicrobial peptide MA-D4 of the present invention, wherein DDDDK is the cleavage site of enterokinase. Then the nucleotide sequence encoding the antimicrobial peptide was designed using E. coli preferred codons, and nucleic acid restriction endonucleases were added at both ends xho and Bam H Identify the site to form the target gene, the sequence of which is shown in SEQ ID No.2.
[0024] The designed target gene sequence is 5′-cac ctcgag gatgatgatgataaacatcatggtattggtaaatttctgcatagcgcaaaaa
[0025] aatttggtaaagca...
Embodiment 2
[0029] Example 2: Acquisition and purification of artificial antimicrobial peptide MA-D4.
[0030] Use enterokinase to digest the expression product according to the following enzyme digestion system: 10×Buffer 10.0 μL, ddH 2 O 38.0 μL, target protein 50.0 μL, enterokinase 2.0 μL, placed at 23°C for 20h.
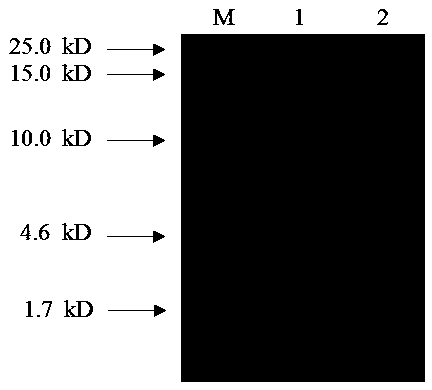
[0031] The artificial antimicrobial peptide MA-D4 of the present invention was obtained by purifying the HisPur Ni-NTA Spin Purification Kit from Thermo Scientific through nickel ion affinity chromatography. After pretreating the resin column according to the instructions, add the enzyme-cut supernatant equal to the amount of High-capacity nickel-IMAC resin, place it in a 4°C environment for 1 hour, elute the protein, and collect the eluate for SDS- PAGE detection, see appendix figure 2 . It can be seen that there is an obvious protein band between 4.6-10.0kD, which is in line with the experimental design size, which is the artificial antimicrobial peptide MA-D4. Its co...
Embodiment 3
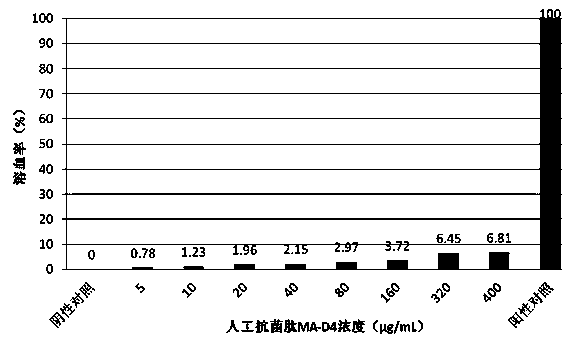
[0032] Example 3: Detection of the biological activity of the artificial antimicrobial peptide MA-D4.
[0033] Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of artificial antimicrobial peptide MA-D4 against E. coli, Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Streptococcus suis type 2 and Bacillus anthracis by microdilution ). The specific experimental steps are as follows: the experimental strains were prepared into 10 6 Cfu / mL bacterial solution was added to 96-well cell culture plates, 90 μL per well. The artificial antimicrobial peptide MA-D4 solution was diluted to 40.0, 20.0, 10.0, 8.0, 6.0, 4.0, 2.0, 1.0, 0.5 μg / mL with PBS, and added to a 96-well cell culture plate, 10 μL per well. The blank control group was set to contain only LB liquid medium, the conditional control group only contained the bacterial liquid of the experimental strain, and the enzyme digestion control group was added with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com