2-methoxy-estradiol analogue and preparation method and application thereof
A technology of methoxyestradiol and analogues, which is applied in the field of medicine, can solve the problems of low water solubility, short half-life, and low solubility, and achieve the effects of simple preparation method, mild conditions, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
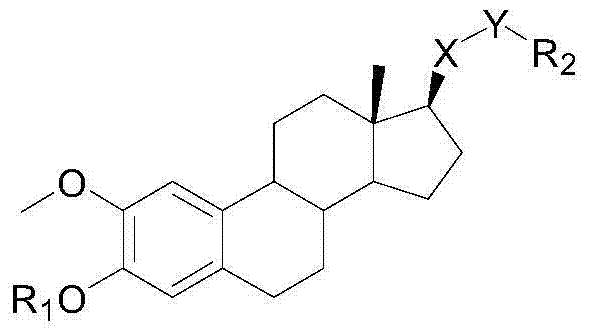
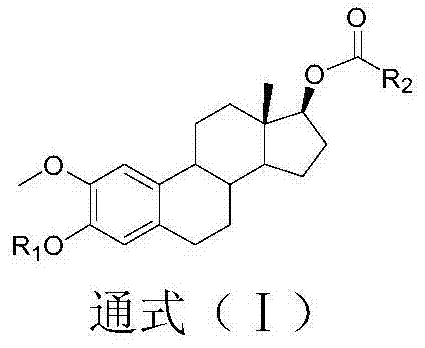
[0023] In the specific implementation of the present invention, the 2-methoxyestradiol analog is a compound of the following structural formula (I):
[0024]
[0025] Among them, R 1It is a kind of hydrogen atom, methyl, ethyl and benzyl; R 2 Methyl, ethyl, vinyl, propyl, isopropyl, propenyl, allyl, butyl, isobutyl, tert-butyl, cyclopentyl, cyclohexane, chloromethyl, chloro Ethyl, chloropropyl, fluoromethyl, fluoroethyl, butyrate, crotonate, hydroxypropionate, phenyl, benzyl, chlorophenyl, fluorophenyl, tetrafluorophenyl, pyridyl and a kind of imidazolyl;
[0026] The compound of the general formula (I) can be one of the compounds of the following general formula:
[0027]
[0028]
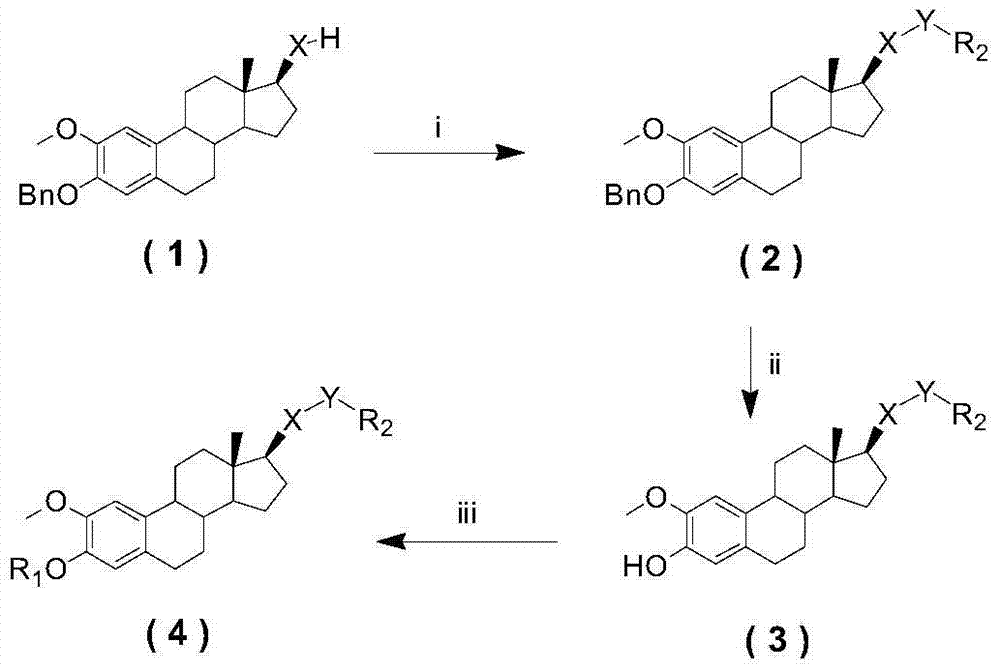
[0029] The preparation method of the compound of the general formula (I), taking the I-3 compound and the I-7 compound as examples:
[0030] The preparation of A, Ⅰ-3 compound, the method is, under the condition of ice bath, add 0.48g (1.22mmol) 2-methoxy-3-benzyloxy-estra-1,3,5(10)-t...
Embodiment 2
[0034] In the specific implementation of the present invention, the 2-methoxyestradiol analog is a compound of the following structural formula (II):
[0035]
[0036] Among them, R 1 hydrogen atom, methyl, ethyl, benzyl, allyl, acetyl, propionyl, butyryl, chloroacetyl, chloropropionyl, chlorobutyryl, benzoyl, chlorobenzoyl, fluorobenzoyl One of acyl and furoyl;
[0037] The compound of the general formula (II) can be one of the compounds of the following general formula:
[0038]
[0039]
[0040] The preparation method of the compound of the general formula (II), taking compound II-4 and compound II-9 as examples:
[0041] A. The preparation of compound Ⅱ-4, the method is to add 169.8 μl (4.5 mmol) of anhydrous formic acid dropwise to 391.8 μl (4.5 mmol) of chlorosulfonyl isocyanate under ice bath, stir rapidly, and add to the system after 15 minutes Acetonitrile 6ml, stirred at room temperature for 7 hours; then slowly added 1.50g (3.8mmol) of 2-methoxy-3-benzyl...
Embodiment 3
[0046] In the specific implementation of the present invention, the 2-methoxyestradiol analog is a compound of the following structural formula (Ⅲ):
[0047]
[0048] Among them, R 1 It is one of hydrogen atom, methyl, ethyl, propyl, allyl, benzyl and aminosulfonyl; R 2 Methyl, ethyl, vinyl, propyl, isopropyl, propenyl, allyl, butyl, isobutyl, tert-butyl, cyclopentyl, cyclohexane, chloromethyl, chloro Ethyl, chloropropyl, fluoromethyl, fluoroethyl, butyrate, crotonate (e.g. fumarate, maleate), hydroxypropionate (e.g. tartaric acid, malate), phenyl, benzyl chlorophenyl, fluorophenyl, tetrafluorophenyl, pyridyl, imidazolyl, natural and unnatural amino acid residues (e.g. glycine, alanine, serine, threonine, valine, leucine , Isoleucine, Phenylalanine, Tyrosine, Cysteine, Methionine, Aspartic Acid, Asparagine, Glutamic Acid, Glutamine, Histidine, Lysine, Arginine , proline, hydroxyproline, tryptophan, β-alanine, γ-aminobutyric acid, ε-aminocaproic acid, ornithine, citrullin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com