N-alkyl-1,2,3,4,5,6-hexahydro-1,1,5,5-tetramethyl-7H-2,4 alpha-methanonaphthalene-7-amine compound as well as synthetic method and application thereof
A technology of endomethylene naphthalene and amine compounds, which is applied in the field of organic compounds, can solve the problems of difficulty in widespread use, few sources of compounds, and low activity, and achieve good effects of inhibiting cancer cell proliferation and activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
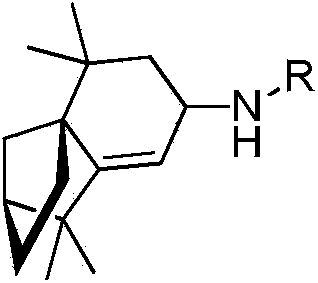
[0042] Example 1 N-hydrocarbyl-1,2,3,4,5,6-hexahydro-1,1,5,5-tetramethyl-7H-2,4α-endomethylenenaphthalene-7-amine compounds preparation of
[0043] The precursor of N-hydrocarbyl-1,2,3,4,5,6-hexahydro-1,1,5,5-tetramethyl-7H-2,4α-methanonaphthalene-7-amines The number of carbon atoms in the skeleton is shown in the following formula.
[0044]
[0045] 1) N-methyl-1,2,3,4,5,6-hexahydro-1,1,5,5-tetramethyl-7H-2,4α-endomethylenenaphthalene-7-amine ( 1) Synthesis of
[0046] With 5.45g isolongifolenone, 15.5g30% (mass percent) methylamine-ethanol solution, 0.3g (accounting for 8% of the isolongifolenone molar number) BF 3 (C 2 h 5 ) 2 Add O and 30mL ethanol to a 100mL three-neck flask equipped with a stirrer, control the temperature at 30°C, and react for 24h under nitrogen protection. GC detects that the imine is about 85.0%, and add 2.72g NaBH in batches at 0°C 4 , react overnight at room temperature after the addition. After adding distilled water to destroy, add ethy...
Embodiment 2
[0075] Embodiment 2 antibacterial test
[0076] 1) The strains to be tested
[0077] Candida albicans (C.albicans), Aspergillus niger (A.niger), Candida tropicalis (G.tropicalis), Escherichia coli (E.coli), Staphylococcus aureus (S.aureus), Pseudomonas fluorescens (P.Fluorescens), Bacillus subtilis (B.subtilis), both provided by Microbiology Laboratory, School of Chemical Engineering, Nanjing Forestry University.
[0078] 2) Preparation of medium
[0079] Beef extract-peptone medium for bacterial culture is prepared as follows: Weigh 5g of beef extract, 10g of peptone, 1g of glucose, 18g of agar, and 1000mL of water, heat and melt, adjust the pH to 7.0-7.2 with 10% sodium hydroxide solution, and pack in Add a cotton plug to the Erlenmeyer flask, autoclave with damp heat (121°C, 20min), and set aside.
[0080] The potato dextrose agar medium (PDA medium) used for fungal culture was prepared as follows: 20 g of potatoes, 20 g of glucose, 18 g of agar, and 1000 mL of water. C...
Embodiment 3
[0090] Embodiment 3 antitumor test
[0091] 1. Cell scratch test
[0092] Tumor is a kind of disease characterized by uncontrolled cell growth and proliferation. Abnormal cell proliferation, differentiation and apoptosis are all involved in the occurrence and development of tumors. As the simplest detection method, cell scratch is used for cell migration research.
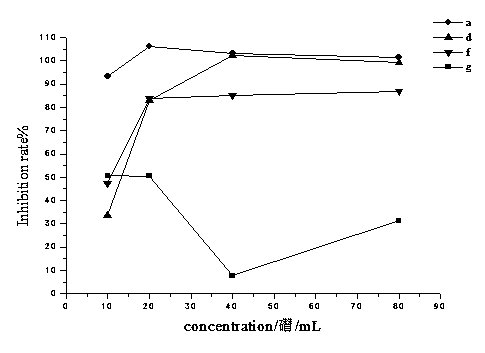
[0093] The inhibitory activity of compounds (1)-(10) on human breast cancer cell MCF-7 was evaluated by cell scratch test. The MCF-7 cells were provided by the Institute of Chinese Materia Medica, Chinese Academy of Chinese Medical Sciences. The specific method is as follows: draw a horizontal line at intervals of about 0.5 to 1 cm on the back of the 6-well plate to ensure that at least 5 lines pass through each well; add about 5×10 5 MCF-7 cells; after overnight, use a pipette tip to draw a horizontal line of "one" on the monolayer cells, wash the cells three times with PBS, remove the marked cells, add serum-f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com