Magnesium lactate production method based on crystallization process for fermentation, separation and coupling
A technology of fermentation separation coupling and magnesium lactate, which is applied in the field of fermentation separation coupling production of magnesium lactate based on the crystallization method, can solve problems such as unfavorable reaction system balance, deterioration of bacterial culture environment, and reduction of product removal rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
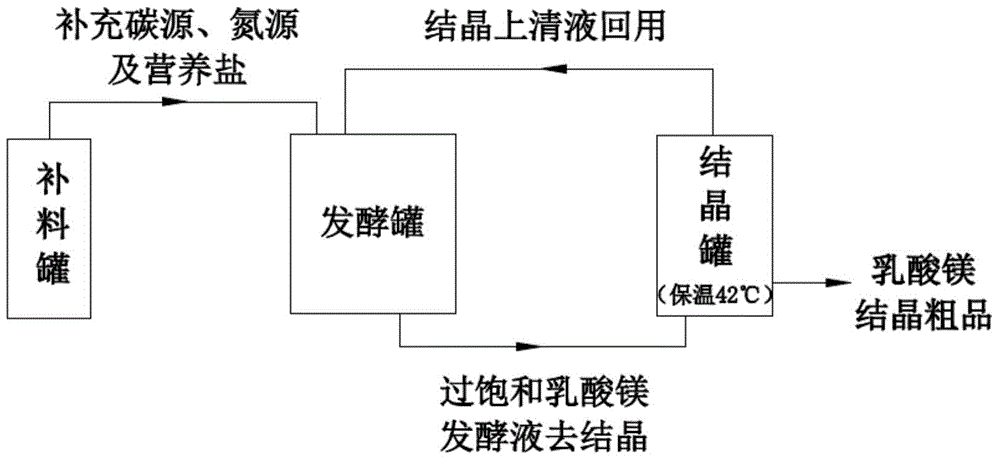
Image
Examples
Embodiment 1
[0055] The medium used in this embodiment is as follows:
[0056] The composition of solid slant medium is (g / L): glucose 20, yeast extract 5, soybean peptone 10, beef extract 10, sodium chloride 10, sodium acetate 5, ammonium citrate 2, magnesium sulfate heptahydrate 0.2, sulfuric acid heptahydrate Manganese 0.05, agar 15. The pH is 6.50.
[0057] The composition of the liquid seed medium is (g / L): glucose 40, peptone 10, yeast extract powder 10, sodium chloride 0.01, sodium acetate 0.5, ammonium citrate 0.2, potassium dihydrogen phosphate 0.4, magnesium sulfate heptahydrate 0.2, Manganese sulfate heptahydrate 0.05. The pH is 6.50.
[0058] The composition of the fermentation medium is (g / L): glucose 150, yeast extract powder 20, sodium chloride 0.01, sodium acetate 0.5, ammonium citrate 0.2, potassium dihydrogen phosphate 0.4, magnesium sulfate heptahydrate 0.2, manganese sulfate heptahydrate 0.05. The pH is 6.00.
[0059] The composition of the feeding medium is (g / L)...
example 2
[0066] 1. Effect of crystallization temperature on crystallization of L-magnesium lactate in fermentation broth
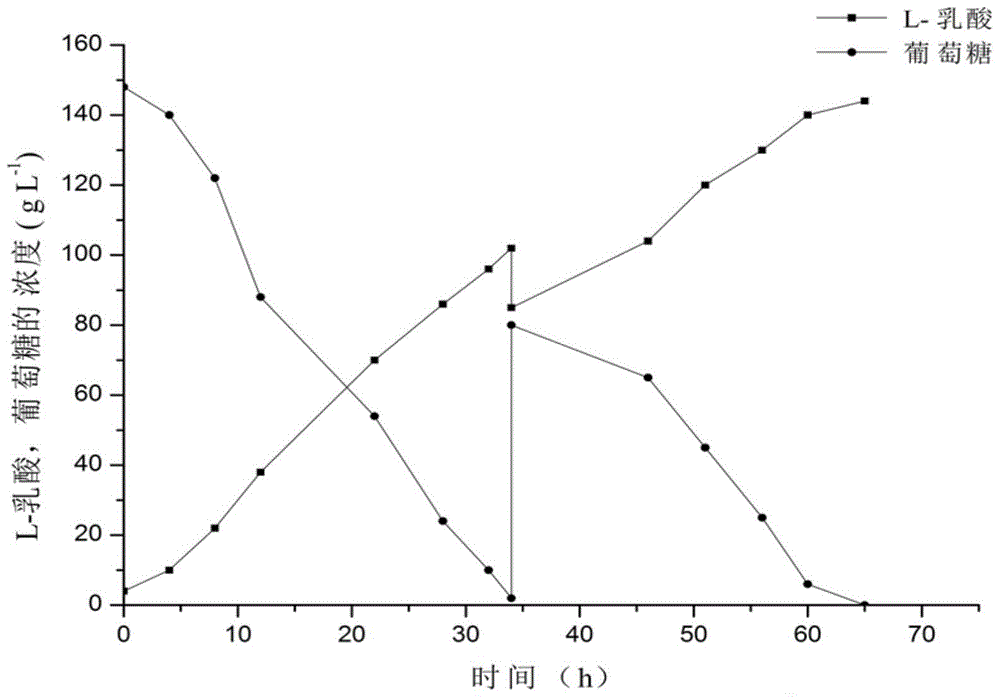
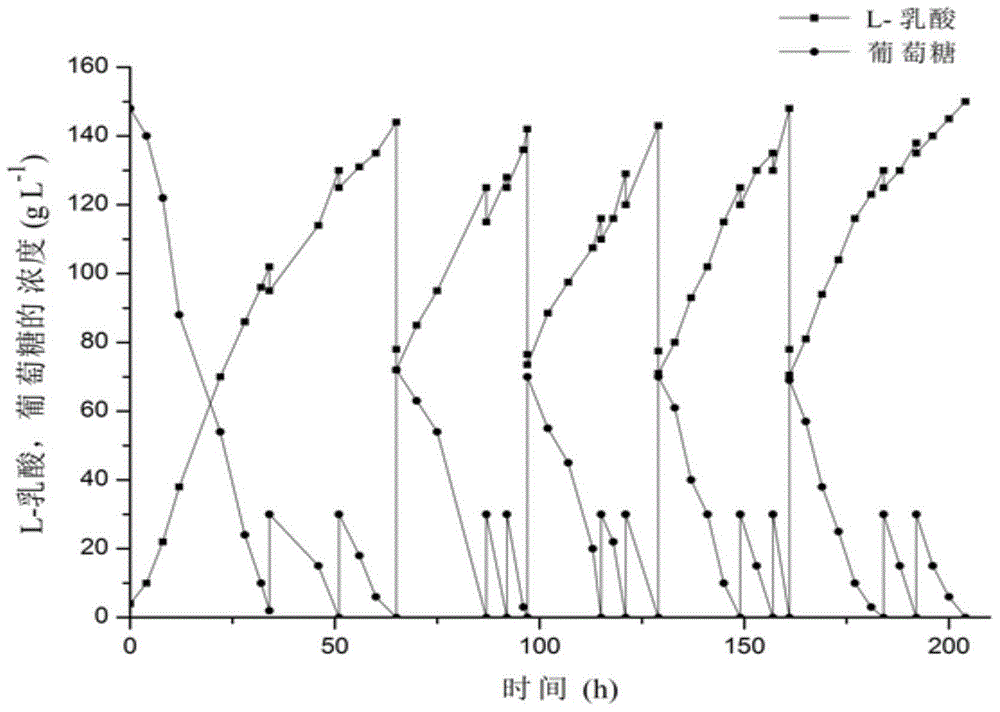
[0067] Obtain the fermented liquid that L-lactic acid content is 140g / L with feeding-batch fermentation mode, fermented liquid is divided into 250ml Erlenmeyer flask by every bottle 100ml, Erlenmeyer flask is placed in different temperatures (temperature gradient is 25-42°C) for 120 minutes, and measure the change of lactic acid content in the supernatant after crystal precipitation at different temperatures, and set up 3 parallel experiments under each temperature condition.
[0068] As shown in Table 1, the concentration of lactic acid in the crystallization supernatant increases with the increase of temperature, and the fitting equation (R 2 =0.996) is:
[0069] C=78.03-0.542T+0.0113T 2 ;
[0070] Where C represents the concentration of lactic acid in the supernatant (g / L), and T represents the crystallization temperature (° C.). As the temperature decreased...
example 3
[0078] The medium used in this embodiment is as follows:
[0079] The composition of the solid slant medium is (g / L): glucose 20, yeast extract 5, soybean peptone 10, beef extract 10, sodium chloride 10, sodium acetate 5, ammonium citrate 2, magnesium sulfate heptahydrate 0.2, sulfuric acid heptahydrate Manganese 0.05, agar 15. The pH is 6.50.
[0080]The composition of the liquid seed medium is (g / L): glucose 40, peptone 10, yeast extract powder 10, sodium chloride 0.01, sodium acetate 0.5, ammonium citrate 0.2, potassium dihydrogen phosphate 0.4, magnesium sulfate heptahydrate 0.2, Manganese sulfate heptahydrate 0.05. The pH is 6.50.
[0081] The composition of the fermentation medium is (g / L): glucose 150, yeast extract powder 20, sodium chloride 0.01, sodium acetate 0.5, ammonium citrate 0.2, potassium dihydrogen phosphate 0.4, magnesium sulfate heptahydrate 0.2, manganese sulfate heptahydrate 0.05. The pH is 6.00.
[0082] The composition of the feeding medium is (g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com