Universal influenza vaccine and preparation method thereof
A kind of influenza vaccine, universal technology, applied in the field of nanovesicle mimicking influenza virus, can solve the problems of chicken embryo death, secondary pollution virus, long production cycle, etc., to achieve large-scale vaccine production, enhance Immunogenicity, the effect of avoiding the production of dangerous viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1V
[0051] The preparation method of embodiment 1 VMV, comprises the following steps:
[0052] (1) 24 hours before transfection, digest the 293T cells in the logarithmic growth phase with 0.25% trypsin at 37°C for 2-3 minutes, and adjust the cell density to 1.2x10 with the medium containing 10% serum. 7 Cells / 20ml, reseeded in 15cm cell culture dish, 37℃, 5%CO 2 Cultured in an incubator. After 24 hours, when the cell density reaches 70%-80%, it can be used for transfection. Cell state is critical for virus packaging, so good cell state and low passage times need to be guaranteed. The cell culture medium was replaced with serum-free medium 2 h before transfection. The HA gene used comes from the H1N1 influenza virus (A / California / 04 / 2009). It is directly obtained by whole gene synthesis.
[0053] (2) Mix the diluted lentiviral packaging plasmid (PLV-HA, PRRE, REV, VSVG, according to the mass ratio 1:1:1:1) and the diluted Lipofectamine2000 (1:2.5), after vigorous shaking, Incu...
Embodiment 2
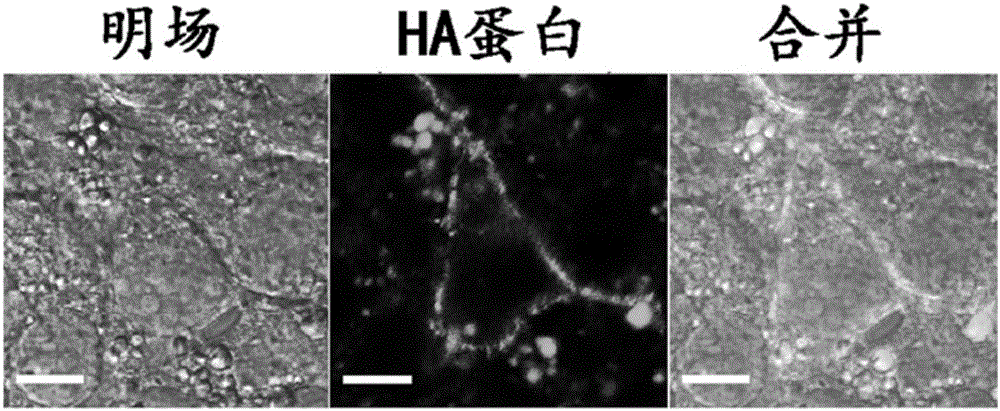
[0059] Example 2 Cellular localization of influenza HA protein, figure 1 :
[0060] Confocal laser microscopy was used to determine the localization of HA protein in eukaryotic cells, while a surfactant was used to promote the production of larger cellular vesicles. Specifically, after the cells were sliced overnight, the PLV-HA plasmid was mixed with liposome2000, then added to the serum-free medium, and replaced with normal medium after 6 hours, and after 48 hours of transfection, the cells were taken out and fixed. , after staining nuclei with DAPI, wash four times with PBS, then add anti-HA mouse monoclonal antibody to incubate for half an hour, after washing, add FITC-labeled goat anti-mouse secondary antibody for immunofluorescence, the final 90% The sections were mounted in glycerol and observed using a Zeiss LSM-780 laser confocal microscope. It can be seen that most of the HA protein is present on the cell membrane, and under the influence of the surfactant (sodiu...
Embodiment 3
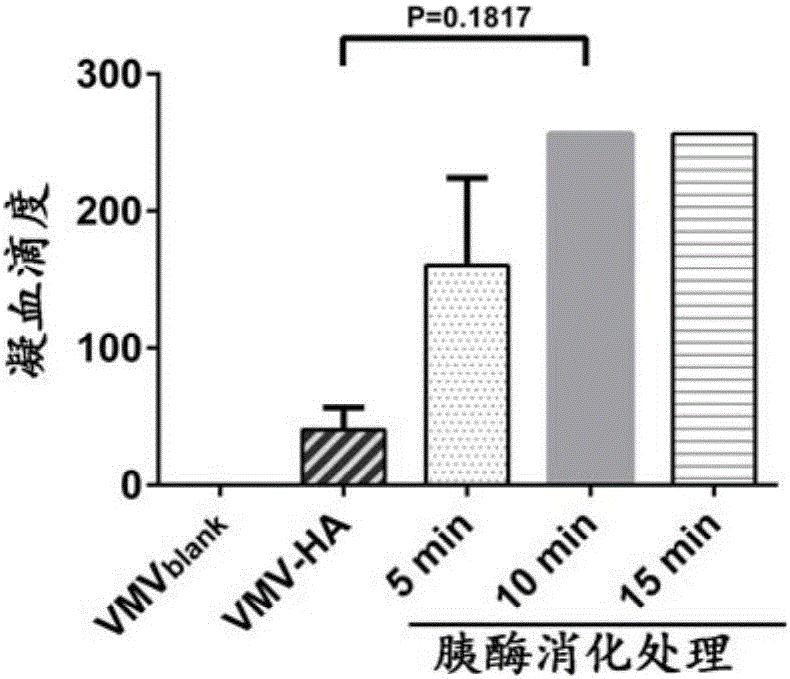
[0063] Example 3. In vitro receptor binding function evaluation (coagulation test) of influenza VMV particles:
[0064] In order to test the in vitro receptor-binding ability of influenza VMV particles, we derived 5×10 5 The VMV of 293T cells was diluted and mixed with 0.5% chicken red blood cells. After standing at room temperature for 40 minutes, the erythrocyte agglutination was observed. After that, there was a significant increase in hemagglutination titer, and this increase has a positive correlation with time and cell number. These data indicate that influenza mimic virus vesicles can bind to cell membrane receptors like normal live viruses, and promote chicken red blood cells. cross-linking ( image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com