Preparation method of microorganism ribosome and application of microorganism ribosome to preparing recombinant protein
A technology of ribosomes and microorganisms, applied in the field of preparation of recombinant proteins, can solve the problems that hinder the extensive research and application of pharmaceutical proteins, difficult to express, and expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] Thermophilus thermophilic culture medium preparation method: take 2g tryptone, 2g yeast extract, 1g sodium nitrate, 0.04g potassium chloride, 0.015g calcium chloride, 0.21g disodium hydrogen phosphate, 0.3g dipotassium hydrogen phosphate , 1.5g of ammonium sulfate and 1ml of trace element solution, dissolved in water to adjust the pH to 7.5, then dilute to 1L with water; sterilize at 121°C for 30min.
[0063] The preparation method of trace element solution (100ml): mix 0.5ml sulfuric acid, 2.28g manganese sulfate, 0.5g zinc sulfate, 0.5g boric acid, 25mg copper sulfate, 25mg sodium molybdate and 45mg cobalt chloride, distilled water to 100ml; Sterilize at 121°C for 30 minutes.
[0064] The preparation method of solid activation medium: compared with the thermophilic bacteria medium, the difference is only to add agar powder and make its mass percentage concentration in the medium be 1% (in practical application, 1%- 1.5% is acceptable).
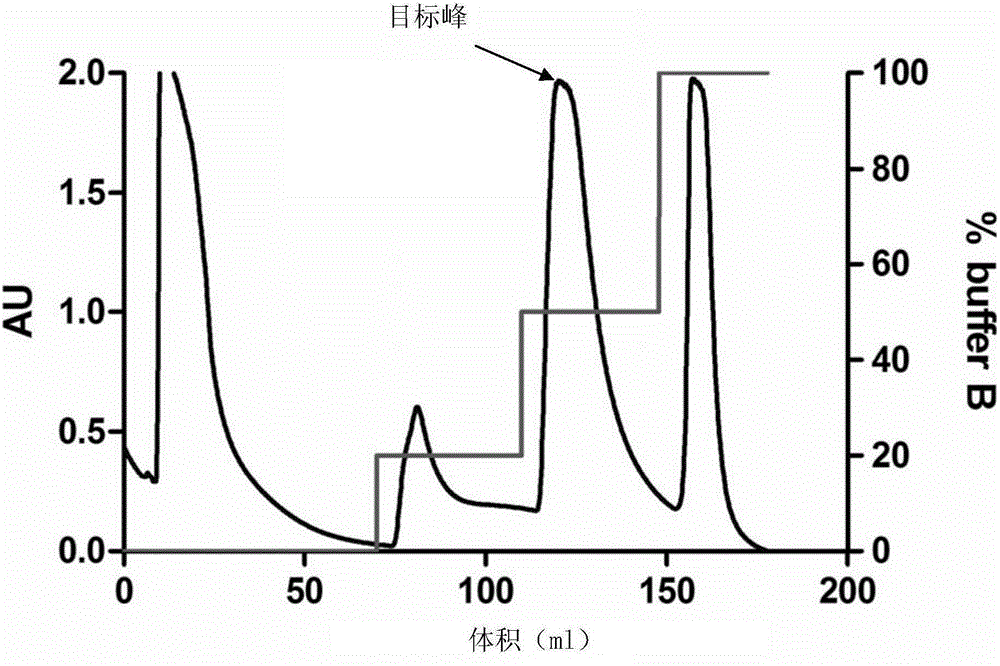
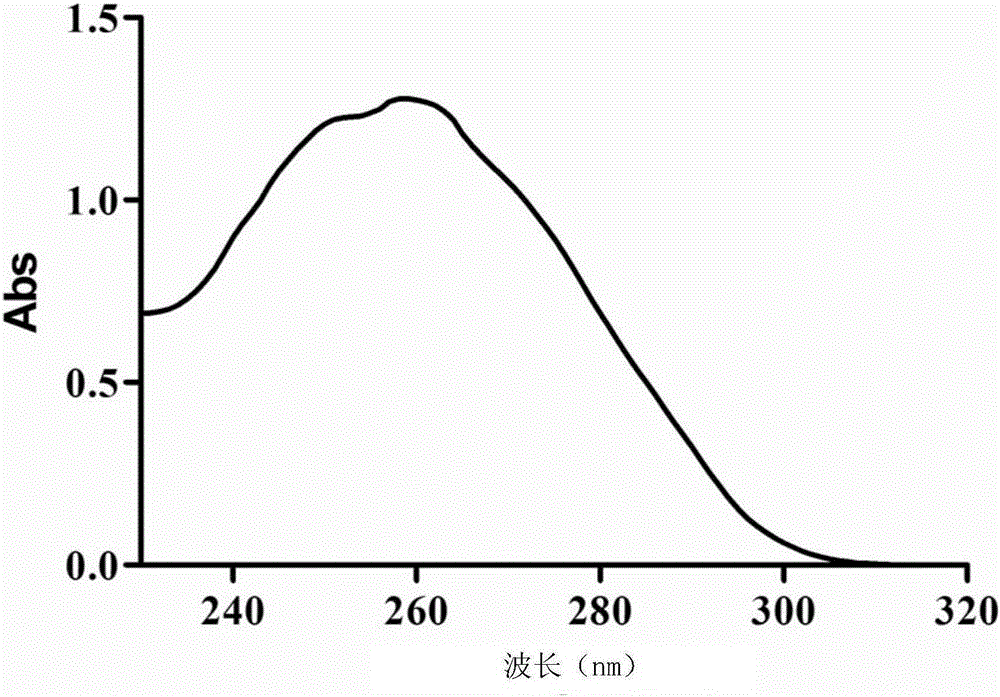
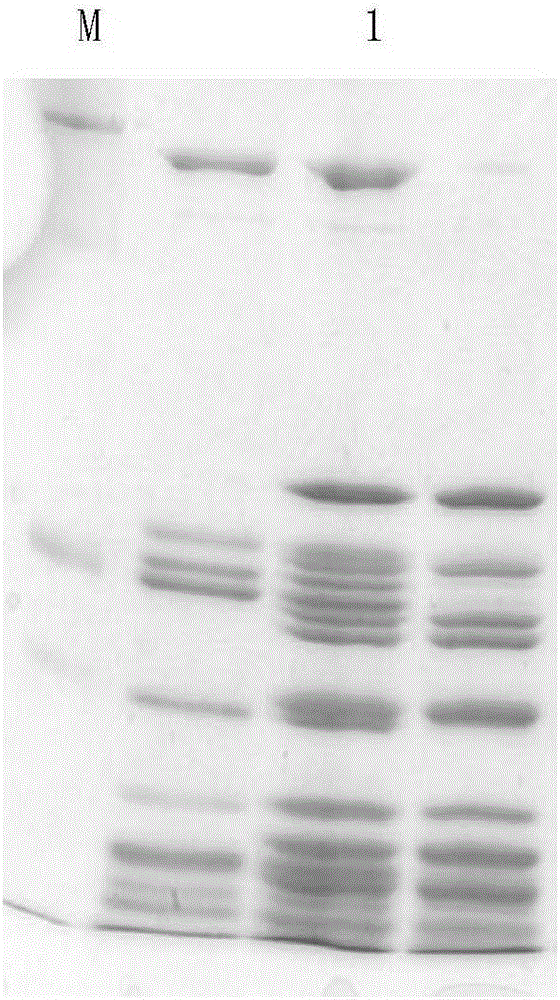
[0065] The preparation metho...
Embodiment 1
[0077] Embodiment 1, the preparation of ribosome
[0078] 1. Expansion of strains
[0079] 1. Take the thermophilic bacteria HB8 cryopreserved in glycerol, inoculate it into a solid activation medium, and culture it statically at 60°C until a single colony grows.
[0080] 2. After completing step 1, pick a single colony, inoculate it into 25ml of Thermus thermophilic culture medium, and culture it with shaking at 60°C and 200r / min for 15 hours (in practical applications, it can be cultivated for 12-18 hours) to obtain seeds liquid.
[0081] 3, get the seed liquid that step 2 obtains, transfer in the 500ml shaking flask that 100ml Thermus thermophiles substratum is housed by 1% inoculum size (1% inoculum size is every 100ml Thermus thermophiles substratum Add 1ml of seed solution to the medium; in practical application, the inoculum size can be 1%-2%, 60°C, 200r / min shaking culture to OD 600nm =2 (in actual application, it is OD600nm =2-3 all can be).
[0082] 4. After comp...
Embodiment 2
[0098] Embodiment 2, utilize the ribosome prepared in embodiment 1 to carry out in vitro synthesis of polyphenylalanine short peptide
[0099] 50 microliters of in vitro synthesis system (test system): the solvent is HEPES-KOH buffer solution with pH 7.6 and 50mM; Phosphoenolpyruvate, 50 μM phenylalanine, 5 μM 14 C-labeled phenylalanine, 2 μM ribosome prepared in Example 1, 2 μg EF-G, 4 μg EF-Tu, 2 μg EF-Ts, 10 μg PheRS, 1.2 μM tRNA Phe (Roche) and 1 mg / ml template. The template is Poly(U) mRNA, as shown in sequence 11 of the sequence listing (that is, 45 consecutive Us), encoding the polypeptide shown in sequence 12 of the sequence listing. A control system A in which ribosomes prepared in Example 1 were replaced by an equal volume of ribosome preservation buffer was set. Set up control system B in which an equal volume of water is used instead of the template.
[0100] The above system was left to react at 37°C for 1 hour, and 8 μl of the reaction solution was taken ever...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com