Clinical-grade placenta mesenchymal stem cell preparation method
A technology of mesenchymal stem cells and placenta, applied in the field of biomedicine, can solve problems such as the influence of cell proliferation ability and cell activity, the complex composition of serum, and the influence of cell adherent growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0089] The invention provides a method for preparing clinical-grade placental mesenchymal stem cells, including the steps of cell separation, primary and passage serum-free expansion, and the like.
[0090] In the present invention, suitable materials include placental tissue materials, usually human placental amnion or chorionic tissue can be selected.
[0091] In the method of the present invention, there is no particular limitation on the volume of the placental tissue material used as a raw material. Typically, the volume is 0.1-5ml, preferably 0.2-2ml, or by weight, 0.1-5g, preferably 0.2-2g.
[0092] The volume ratio of the placental amnion or chorion tissue of the present invention to the combined digestive fluid of the present invention is not particularly limited, usually 1g:2-4ml (weight / volume ratio).
[0093] In the present invention, the digestion conditions with combined enzymes include: digestion at 37±2°C; and / or digestion for 6-24 hours, preferably 8-20 hours...
Embodiment 1
[0138] Preparation of primary placental mesenchymal stem cells
[0139] 1. The placental sample is qualified after strict infectious virus testing (hepatitis B virus, hepatitis C virus, HIV, syphilis and cytomegalovirus) and the result is negative, and enters the cell separation process.
[0140] 2. In a class 100 clean environment, such as an ultra-clean workbench, cut the placental amniotic membrane or chorionic tissue into 1% penicillin / streptomycin PBS solution, remove the remaining blood stains, cut it into pieces mechanically with scissors, and collect In a 15ml centrifuge tube, add 1:3-4ml volume ratio (80U / ml, 0.008wt% working concentration of collagenase type IV, trypsin) combined digestion solution; after digestion at 37°C for 12-18h, preferably 16h, fully blown Mix well, transfer the supernatant suspension to a new 15ml centrifuge tube, centrifuge at 300-500g for 5min, and harvest the cell pellet.
[0141] 3. Add 1-2ml red blood cell lysate to the cells harvested i...
Embodiment 2
[0145] Serum-free subculture, harvest, and passaging of primary cells
[0146] Add the primary placental mesenchymal stem cells obtained in Example 1 to resuspend in serum-free medium, press 1:3-6 (cell number dilution ratio) or 3000-6000 cells / cm 2 Density passage expansion.
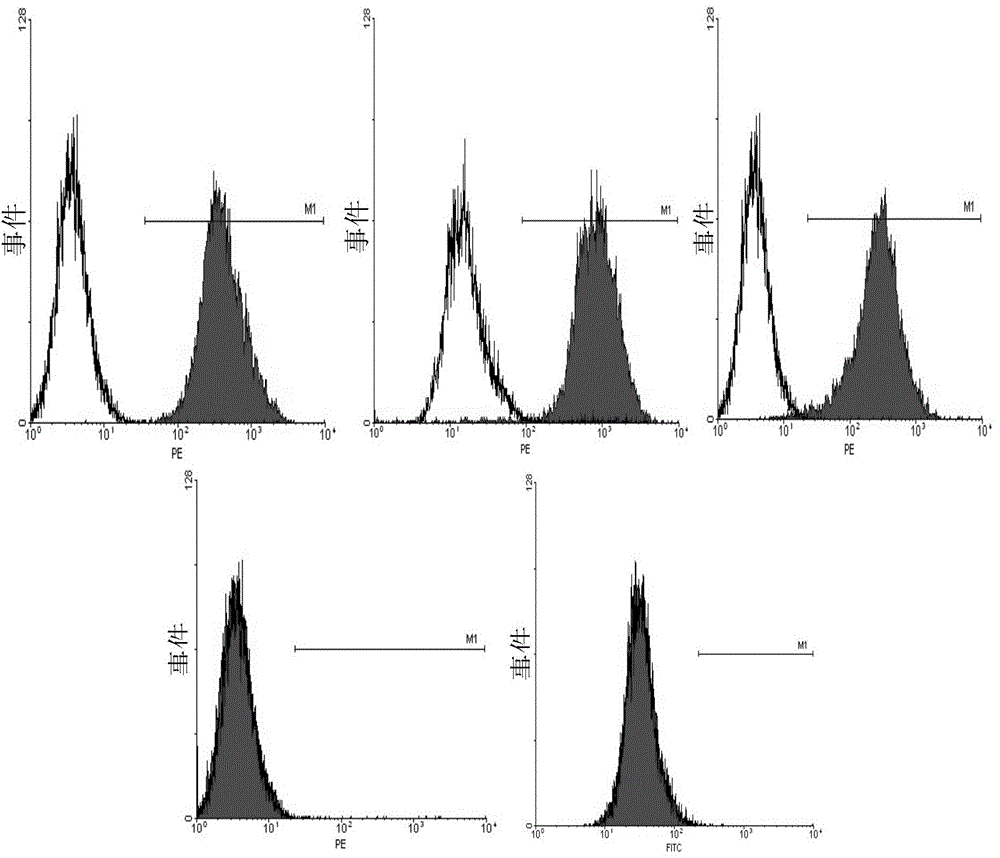
[0147] After 1-2 amplification passages, low-passage, 10 7 Cells of the above order of magnitude were stored at -196°C, and the cells were analyzed by flow cytometry for surface markers.
[0148] Results of cell morphology identification
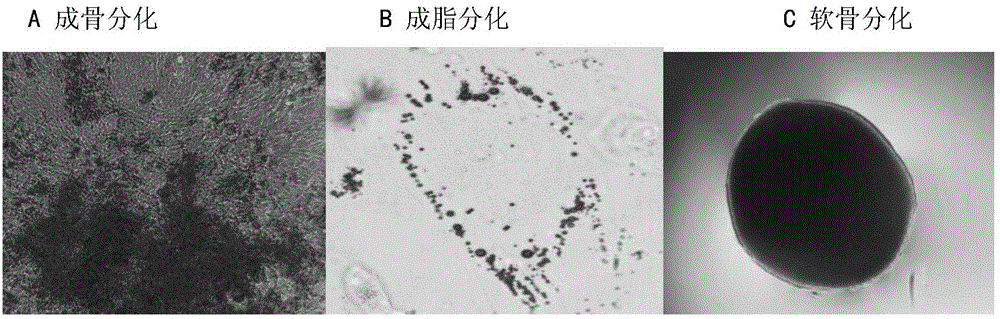
[0149] Cell morphology was observed on the amplified cells, and the results were as follows:
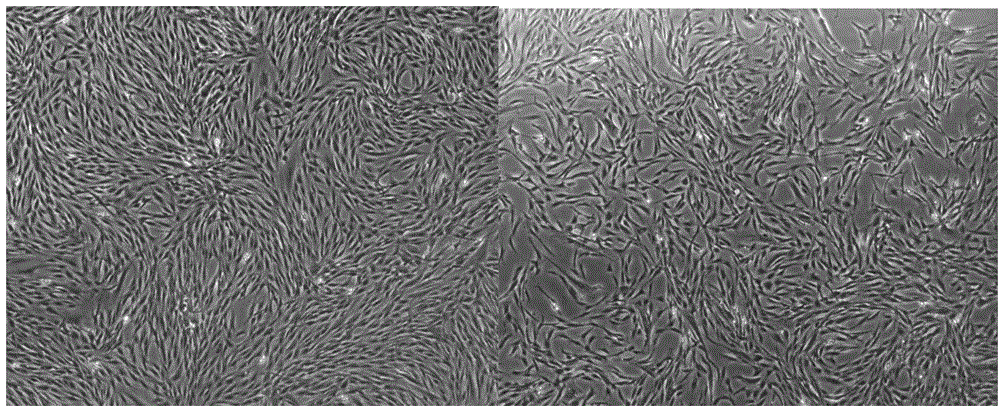
[0150] Placental mesenchymal stem cells obtained by the separation and serum-free expansion method. The morphology under the inverted microscope is as attached figure 1 As shown, the adherent cells of the P5 generation were spindle-shaped and polygonal, with uniform cell shape and size and strong cell proliferation ability.
[0151] Flow Cytometry Surface Marker Anal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com