Amphiphilic aza-BODIPY fluorescent dye and preparation method thereof
A technology of fluorine boron dipyrrole and fluorescent dyes, which is applied in the direction of azo dyes, organic dyes, luminescent materials, etc., can solve the problem of short ultraviolet absorption wavelength, and achieve the effect of sharp peak shape and high intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 (C 30 h 38 o 3 )
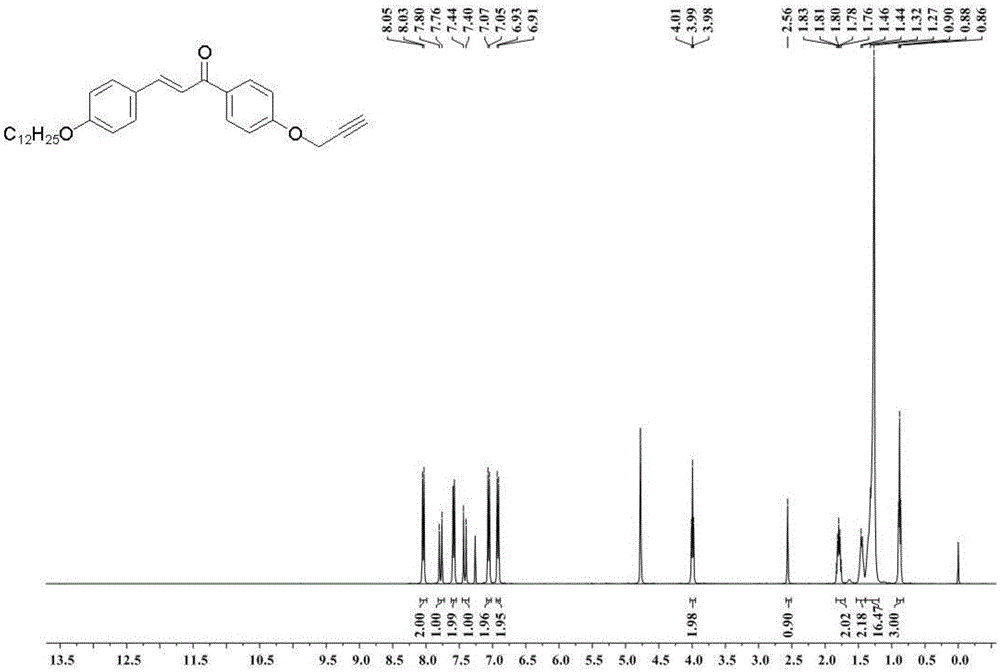
[0037] 4'-(3-propynyloxy)-4-(dodecyloxy)-chalcone (C 30 h 38 o 3 ) synthesis: p-propynyloxyacetophenone 15mmol is dissolved in the mixed solution of ethanol 50ml containing potassium hydroxide 60mmol and water 15ml, stirred for 30min, and the ethanol solution containing p-dodecyloxyphenylacetaldehyde 15mmol is slowly added dropwise , reacted at room temperature for 12h, filtered to obtain a precipitate, and washed with water to neutral pH to obtain a white powdery solid. 1 HNMR: (400MHz, CDCl 3 ):8.05(d,J=8.8Hz,2H),7.80(d,J=15.6Hz,1H),7.60(d,J=8.5Hz,2H),7.44(d,J=15.6Hz,1H), 7.07(d,J=8.7Hz,2H),6.93(d,J=8.6Hz,2H),4.78(d,J=2.1Hz,2H),3.99(t,2H),2.56(s,1H)1.80 (m,2H),1.46-1.27(m,18H),0.90(t,3H); image 3 shown.
[0038]
Embodiment 2
[0039] Embodiment 2 (C 62 h 74 BF 2 N 3 o 4 )
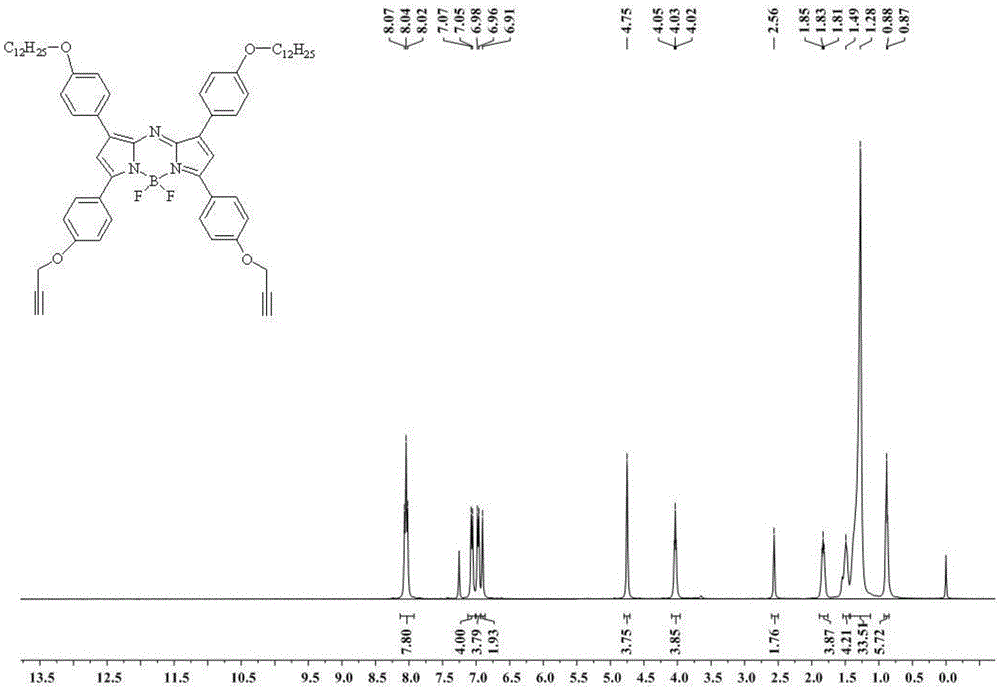
[0040]1,7-(4-dodecyloxy)phenyl-3,5-(4-propynyloxy)phenylazafluoroborondipyrrole (C 62 h 74 BF 2 N 3 o 4 ) Synthesis of fluorescent dye: 12 mmol of 4'-(3-propynyloxy)-4-(dodecyloxy)-chalcone and 400 mmol of ammonium acetate were mixed, refluxed for 12 hours, 200 ml of water was added, and 200 ml of dichloromethane was extracted Three times, dried over anhydrous sodium sulfate, filtered, and the filtrate was rotary evaporated to remove the organic solvent.
[0041] Add 50ml of dry dichloromethane to the obtained compound under nitrogen atmosphere, add DIPEA 10mmol, add 12mmol boron trifluoride ether solution after 30min, and react at room temperature for 24h. Sequentially add saturated ammonium chloride solution, sodium chloride solution, and water to extract separately, the obtained organic solvent is dried with anhydrous sodium sulfate, and the obtained filtrate is filtered to remove the organic solvent by rotary evapor...
Embodiment 3
[0043] Embodiment 3 (C 72 h 96 BF 2 N 9 o 8 )
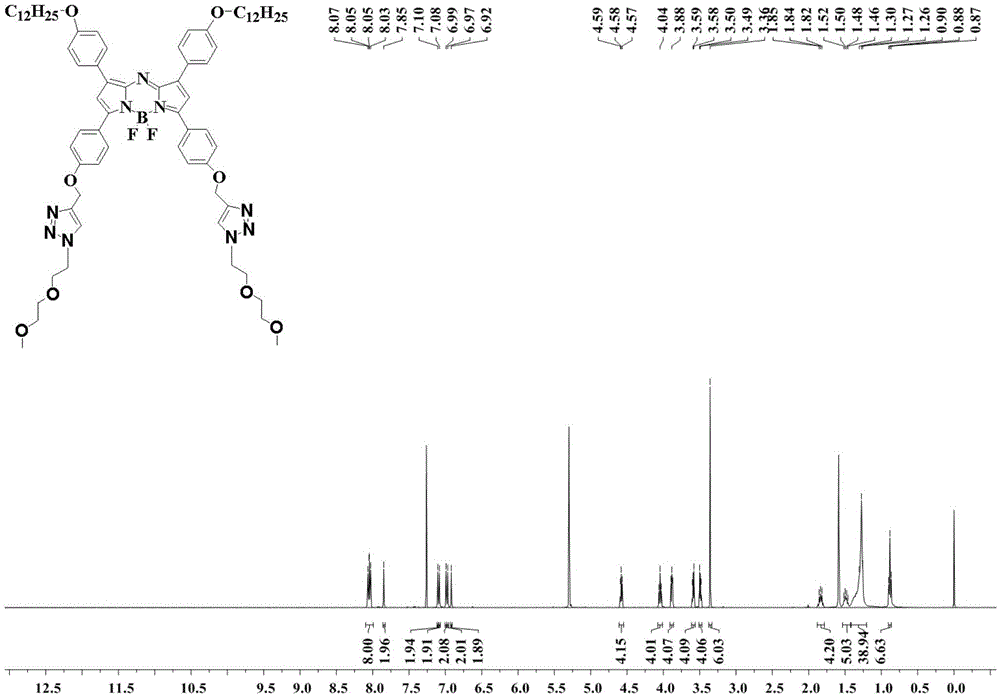
[0044] 1,7-(4-dodecyloxy)phenyl-3,5-(4-propynyloxy)phenylazafluoroboron dipyrrole 0.1mmol, azide diethylene glycol methyl ether 0.23mmol , 0.4mmol of copper sulfate pentahydrate, 0.5mmol of L-ascorbic acid were successively added into a mixed solvent of 30ml of acetonitrile, 30ml of tetrahydrofuran and 2ml of water, and stirred at room temperature for 24h. The organic solvent was removed by rotary evaporation, dichloromethane was added to dissolve and washed three times with water, the obtained organic solvent was dried with anhydrous sodium sulfate, and the obtained filtrate was filtered to remove the organic solvent by rotary evaporation. The product was obtained by preparative column chromatography, and the volume ratio of the eluent was dichloromethane:methanol=20:1. 1 HNMR: (400MHz, CDCl 3 ):8.07(m,8H),7.85(s,2H),7.10(d,j=9.0Hz,4H),6.99(d,j=8.9Hz,4H),6.92(s,2H),4.59(t ,4H),4.06(t,4H),3.90(t,4H),3.60(m,4H),3.50(m,4H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com