Novel gene engineering recombinant TRAIL fusion protein, and preparation method and use thereof
A fusion protein and genetic engineering technology, applied in the field of new genetic engineering recombinant TRAIL fusion protein and preparation, can solve the problems of weak biological activity, poor stability, short half-life of TRAIL, etc., achieve high purity, simple method, good anti-tumor or The effect of cancer cell activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
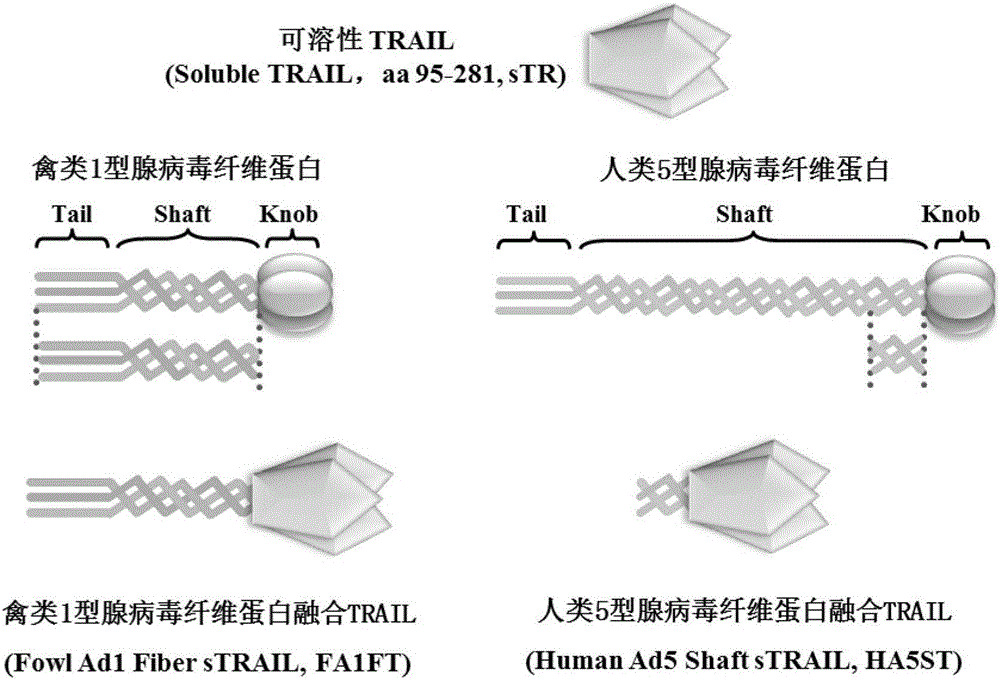
[0031] Example 1 Construction of Adenovirus Fiber-TRAIL Fusion Protein Prokaryotic Expression Vector
[0032] 1. Obtaining the target gene
[0033] PCR was carried out using the plasmid pDC316-TRAIL (human, aa95-281) with the TRAIL gene stored in the laboratory as a template, and the reaction conditions were: pre-denaturation at 95°C for 10 minutes; denaturation at 95°C for 30 seconds, annealing at 56°C for 30 seconds, and extension at 72°C 1min, 30 cycles; 72°C extension for 10min. PCR products were identified by 1.2% agarose gel electrophoresis. Agarose gel recovery kit for purification of PCR products. Use Vector NTI Suited 6 software according to the human TRAIL gene sequence TRAIL primer that Genebank provides as follows:
[0034] Upstream primer sequence: 5'- GCTAGC ATGACCTCTGAGGAAACCATTTCTAC-3', containing NheI restriction site.
[0035] Downstream primer sequence: 5'- AAGCTT TTAGCCAACTAAAAAGGCCC-3', containing Hind IIl restriction site.
[0036] 2. Constructio...
Embodiment 2
[0046] Example 2 Construction of Adenovirus Fiber-TRAIL Fusion Protein Prokaryotic Expression Vector
[0047]1. Prokaryotic expression
[0048] The above three prokaryotic expression plasmids were transformed into Escherichia coli BL21 (DE3) competent cells (Dalian Bao Biology), spread on LB solid medium containing kanapenicillin (100ug / ml), and placed in a 37°C incubator Incubate overnight. Pick a single colony from the above plate and inoculate it in 10mL LB liquid medium for overnight culture. The obtained bacterial liquid was inoculated into 1L LB liquid medium, and cultured in large quantities by shaking at 37°C. When the OD600 value of the bacteria reached 0.8, the inducer isopropyl-β-D-thiogalactoside (IPTG, Beijing Dingguo) was added at a final concentration of 1 mmol / L, and the expression was induced at 20°C for 16 hours. SDS-PAGE electrophoresis was performed on the bacterial cell samples before and after induction, such as image 3 .
[0049] 2. Protein purific...
Embodiment 3
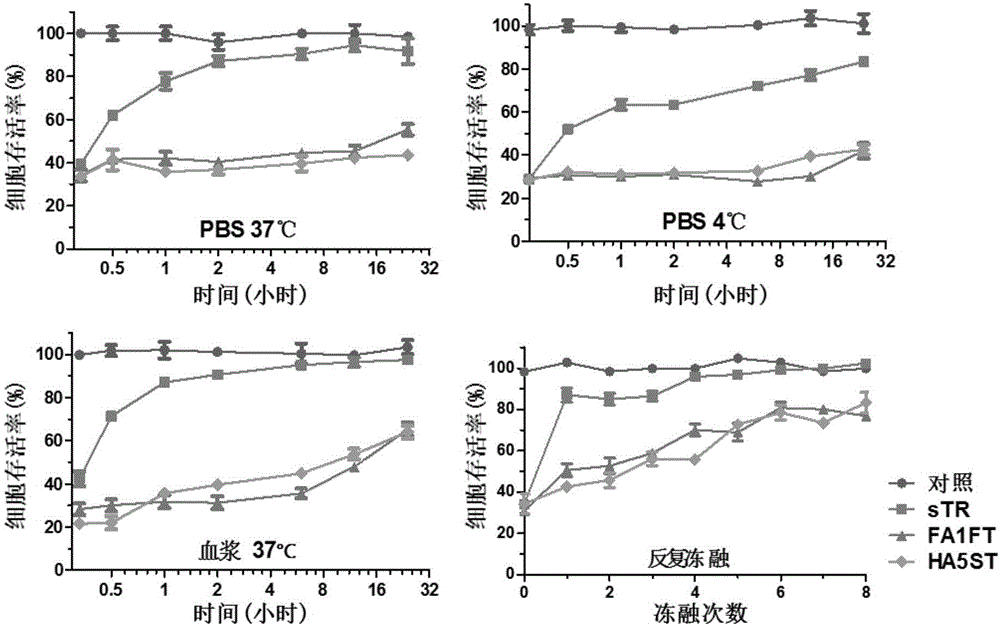
[0055] Example 3 Detection of the Killing Effect of Fiber-TRAIL Fusion Protein on Tumor Cells
[0056] Tumor cell lines:
[0057] Breast cancer cells: ZR-75-30, MCF7; lung cancer cells: A549; liver cancer cells: SMMC-7721; colon cancer cells: SW480; cervical cancer cells: Hela; normal breast cells: MCF-10A; normal liver cells: Chang Liver .
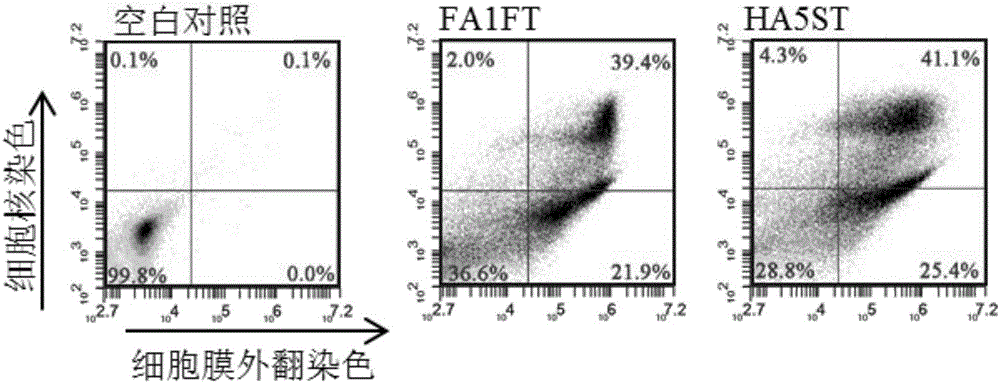
[0058] 1) The killing effect of FA1FT and HA5ST on tumor cells
[0059] Experimental method: Cells were cultured in a medium containing 10% calf serum, 100μg / ml streptomycin and 100U / ml penicillin at 37°C, 5% CO 2 cultivated under conditions. Will 1×10 4 3 cells were inoculated in 96-well plates, adhered to the wall overnight, then the culture medium was changed to the medium containing 2% calf serum, and the mixture obtained in Example 2 with different concentration gradients (0.01, 0.1, 1, 10 and 100 nM) was added. fusion protein. After reacting overnight, 20 μl of MTT solution was added, and after 4 hours of reaction, the superna...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com