Umbilical cord mesenchymal stem cell injection liquid, preparation method and application thereof
A stem cell and injection technology, applied in the field of stem cells, can solve the problems of less application of acute rejection, painful economic burden for patients, low immunogenicity, etc., and achieve the effects of easy industrial preparation, long storage time, and reduced incidence.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
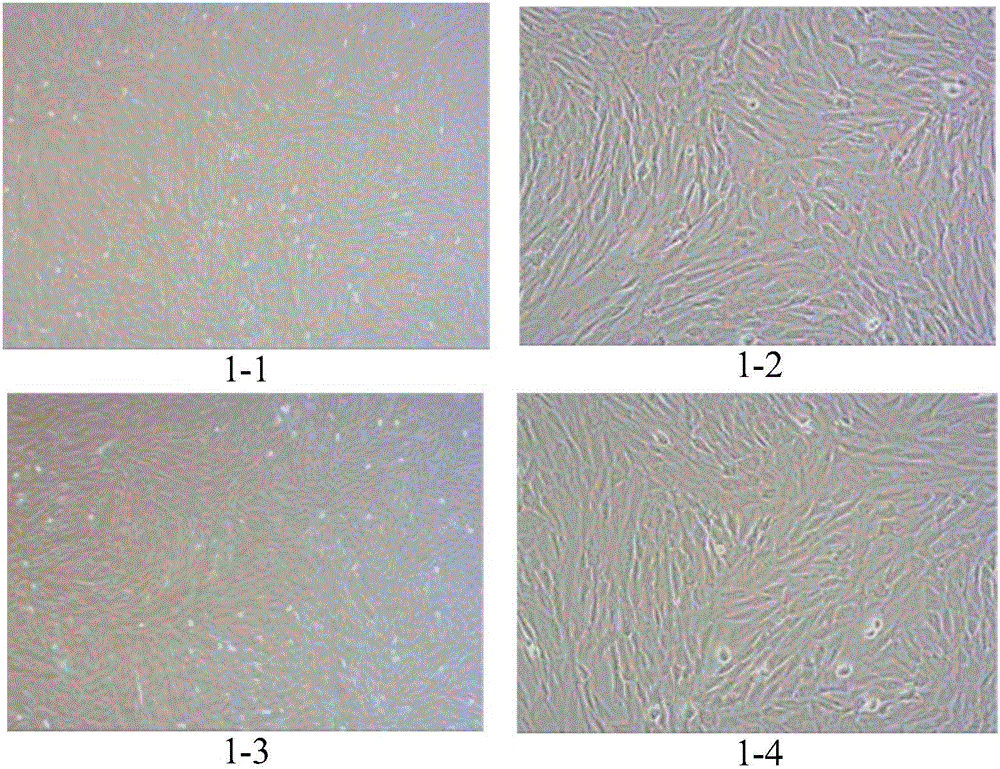
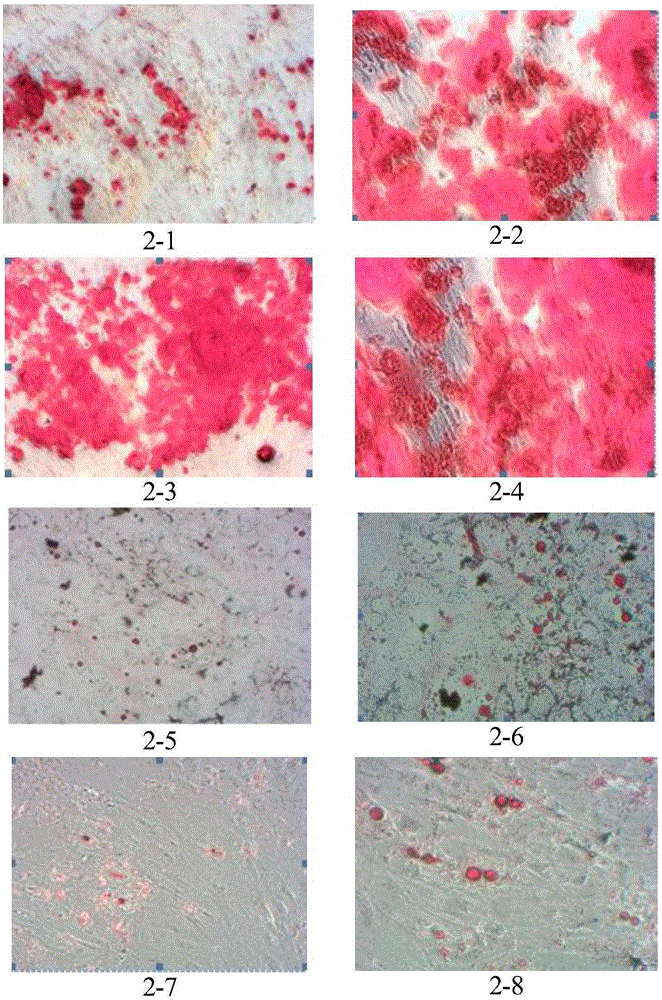
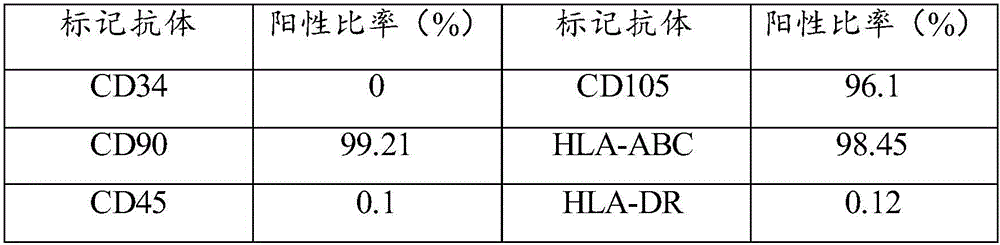
[0068] Example 1 Preparation method of umbilical cord mesenchymal stem cells
[0069] Under sterile conditions, take out the fresh umbilical cord that has been away from the mother for no more than 2 hours, and screen whether the mother is carrying infectious disease pathogens (negative for hepatitis B surface antigen, hepatitis C antibody, syphilis antibody, and AIDS antibody), and place the fresh umbilical cord in a double Antibiotic PBS was transported back to the laboratory in a 4°C incubator.
[0070] Clean the fresh umbilical cord with sterile PBS, then disinfect it with 75% ethanol, and then clean it with PBS. Finally, cut the umbilical cord to 1~3cm 3 Evenly inoculate the pieces with the size of 0.5 ~ 1cm in the petri dish, slowly add 50μL ~ 100μL / cm 2 Complete medium, placed at 37°C, CO 2 The concentration was 5% in the incubator for static culture.
[0071] After the cells adhere to the wall and fuse, digest with trypsin, centrifuge, divide into multiple culture ...
Embodiment 2
[0084] Prepare 100mL stem cell injection: 30mL of human serum albumin stock solution with albumin concentration of 10% (final concentration of mass volume ratio is 3%), low molecular weight heparin calcium 0.1mL (volume percentage content is 0.1%), vitamin C 0.5 g (mass volume ratio final concentration is 0.5%), pearl ginsenoside 12.5g (mass volume ratio final concentration is 12.5%) and 0.9% sodium chloride injection 69.9mL are mixed, and the collected P5 generation umbilical cord mesenchymal stem cells (final cell concentration 1×10 6 individual / mL) in the mixed solution, resuspended in The cells are stored in the cryopreservation bag, and the preparation is complete.
[0085] The reserved samples of the umbilical cord mesenchymal stem cell injection prepared above are tested for endotoxin, bacteria and fungi, and mycoplasma. If the test results are all negative, it is qualified.
Embodiment 3
[0087] Prepare 100mL stem cell injection: 5mL of human serum albumin stock solution with albumin concentration of 20% (final concentration of mass volume ratio is 1%), low molecular weight heparin calcium 0.1mL (volume percentage is 0.1%), vitamin C 0.3 g (mass volume ratio final concentration is 0.3%), pearl ginsenoside 5g (mass volume ratio final concentration is 5%) and 0.9% sodium chloride injection 94.9mL are mixed, and the collected P5 generation umbilical cord mesenchymal stem cells ( Cell final concentration 1×10 6 individual / mL) in the mixed solution, resuspended in The cells are stored in the cryopreservation bag, and the preparation is complete.
[0088] The reserved samples of the umbilical cord mesenchymal stem cell injection prepared above are tested for endotoxin, bacteria and fungi, and mycoplasma. If the test results are all negative, it is qualified.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com