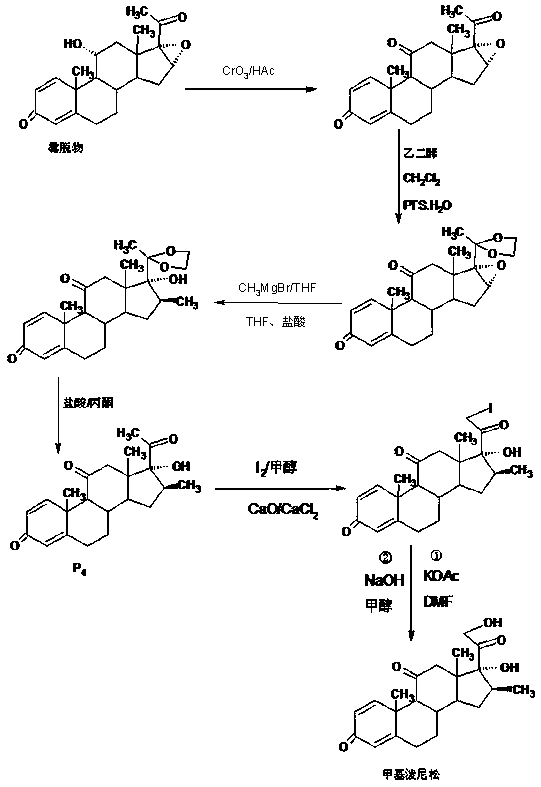
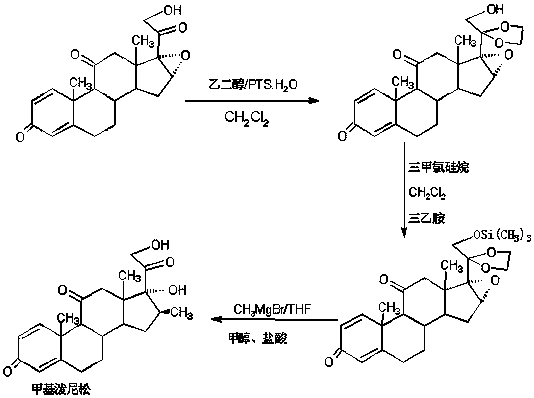
The preparation method of methylprednisone
A technology of methylprednisone and epoxyprednisone, which is applied in the direction of steroids and organic chemistry, can solve the problems of complex process operation, difficult sewage treatment, high production cost, etc., and achieve process economy and environmental protection, and reduce production cost , The effect of easy production and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A: Preparation of ketals
[0021] In a 1000ml three-necked bottle, add 100g 16(17)a-epoxyprednisone, 150ml ethylene glycol, 80ml triethyl orthoformate, 2g p-toluenesulfonic acid, keep warm at 20-25℃ and stir for 16~ After 18 hours, TLC detects the end point of the reaction. After the reaction, add 2ml of triethylamine to neutralize to a pH of about 7, then concentrate under reduced pressure to recover 90% of the solvent, cool, add 500ml of tap water, stir and crystallize for 60-90 minutes, Centrifugation, washing, rejection filtration, discharge of the filtrate and lotion to the wastewater treatment tank, the filter cake is directly recrystallized with 20% ethanol aqueous solution, and dried to obtain the ketal product: 20-ketal-16(17)a-epoxyprednisol Pine 112g, HPLC content 98.0%, weight yield 112%.
[0022] B: Preparation of silicon ethers
[0023] In a 1000ml three-neck flask, add 100g of ketal, 400ml of dichloromethane, 80ml of trimethylchlorosilane, add 30g of et...
Embodiment 2
[0027] A: Preparation of ketals
[0028] In a 1000ml three-necked bottle, add 100g 16(17)a-epoxyprednisone, 400ml dichloromethane, 40ml ethylene glycol, 80ml triethyl orthoformate, 2g p-toluenesulfonic acid, keep warm at 20-25 Stir and react at ℃ for 16-18 hours, TLC detects the end point of the reaction, after the reaction, add 2ml triethylamine to neutralize to pH about 7, then concentrate under reduced pressure to recover 90% of the solvent, cool, add 500ml tap water, stir and crystallize for 60 After -90 minutes, centrifuge, wash, and filter by rejection. The filtrate and washing liquid are discharged to the waste water treatment tank, and the filter cake is directly recrystallized with 20% ethanol aqueous solution, and dried to obtain the ketal product: 20-ketal-16(17)a - epoxy prednisone 110g, HPLC content 98.5%, weight yield 110%.
[0029] B: Preparation of silicon ethers
[0030] In a 1000ml three-neck flask, add 100g of ketal, 400ml of chloroform, 80ml of trimethylc...
Embodiment 3
[0034] A: Preparation of ketals
[0035] In a 1000ml three-neck flask, add 100g 16(17)a-epoxyprednisone, 400ml toluene, 40ml ethylene glycol, 80ml triethyl orthoformate, 2g p-toluenesulfonic acid, keep stirring at 20-25°C React for 16-18 hours, TLC detects the end point of the reaction, after the reaction, add 2ml triethylamine to neutralize to a pH of about 7, then concentrate under reduced pressure to recover 90% of the solvent, cool, add 500ml tap water, stir and crystallize for 60-90 Minutes later, centrifuge, wash, reject and filter, and the filtrate and washing liquid are discharged to the waste water treatment tank, and the filter cake is directly recrystallized with 20% ethanol aqueous solution, and dried to obtain the ketal product: 20-ketal-16(17)a-ring Oxyprednisone 110.6g, HPLC content 97.5%, weight yield 110.6%.
[0036] B: Preparation of silicon ethers
[0037] In a 1000ml three-neck flask, add 100g of ketal, 400ml of dichloromethane, and 80ml of trimethylchlor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com