Anticoagulation high polymer biomaterial as well as preparation method and application thereof
A biomaterial, anticoagulant technology, applied in the field of biomedical engineering, can solve the problems of heparin conformational limitation, reduced anticoagulant activity, difficult anticoagulant materials, etc., to ensure stability, durability, coating good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
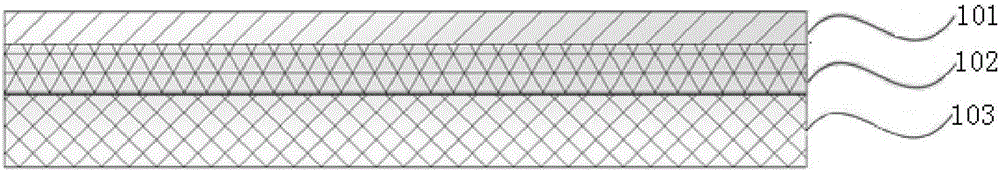
[0039] Such as figure 1 and figure 2 As shown, the anticoagulant polymer biomaterial of the present invention includes the following three-layer structure: a base layer, an anticoagulant coating, and a plasma polymerized layer between the base layer and the anticoagulant coating; wherein,
[0040] The base layer is made of biomedical materials, the biomedical materials include synthetic polymer materials and natural polymer materials, wherein the synthetic polymer materials include polyurethane, silica gel and other organic silicon polymers, organic glass, nylon, polyester, polytetrafluoroethylene Vinyl fluoride, silicone rubber, polyester fiber, polyvinylpyrrolidone, polyether ether ketone, polymethyl methacrylate, polyvinyl alcohol, polylactic acid, polyethylene, etc.; natural polymer materials include chitosan, collagen, silk protein, cellulose, etc.
[0041] The plasma polymerized layer is a layer of acrylamine plasma polymerized film deposited on the base layer;
[00...
Embodiment 2
[0046] Preparation of polycarbonate surface anticoagulant polymer biomaterial based on Example 1
[0047] Put the base material (polycarbonate) into a vacuumed plasma gas generator, the air pressure is 0.05 Pa, adjust the power of the radio frequency generator to 500W, and under the condition of frequency 10 MHz, pretreat the surface of the base material to clean and oxidize the surface. The treatment time is 0.01h; then under the same conditions, use acrylamide vapor for 0.1h to deposit a layer of acrylamine plasma polymerized film on the substrate material; finally, place the plasma-treated substrate material in 10mg / ml heparin The prepared sodium cyanoborohydride buffer solution was placed on a shaker at room temperature for 12 hours, then washed with PBS, and dried to obtain an anticoagulant polymer biomaterial. The structure of anticoagulant molecules on the surface of the substrate material is as follows: figure 1 shown.
Embodiment 3
[0049] Preparation of the silica gel surface anticoagulant polymer biomaterial based on Example 1
[0050] Put the base material (silica gel) into a vacuumed plasma gas generator, the air pressure is 0.01 Pa, adjust the power of the radio frequency generator to 1000W, and the frequency is 180 MHz, pretreat the surface of the base material, clean and oxidize the surface, and the treatment time 0.02h; then under the same conditions, use acrylamide vapor treatment for 0.15h to deposit a layer of acrylamine plasma polymerized film on the substrate material; finally, put the plasma treated substrate material placed in a sodium cyanoborohydride buffer solution at room temperature for 24 hours, then washed with PBS, and dried to obtain an anticoagulant polymer biomaterial.
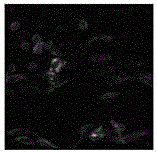
[0051] The coated anticoagulant polymer biomaterial was sterilized and implanted into mice, and the implantation site was taken out after 2 weeks and 4 weeks of culture, and cell immunological staining was perfor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com