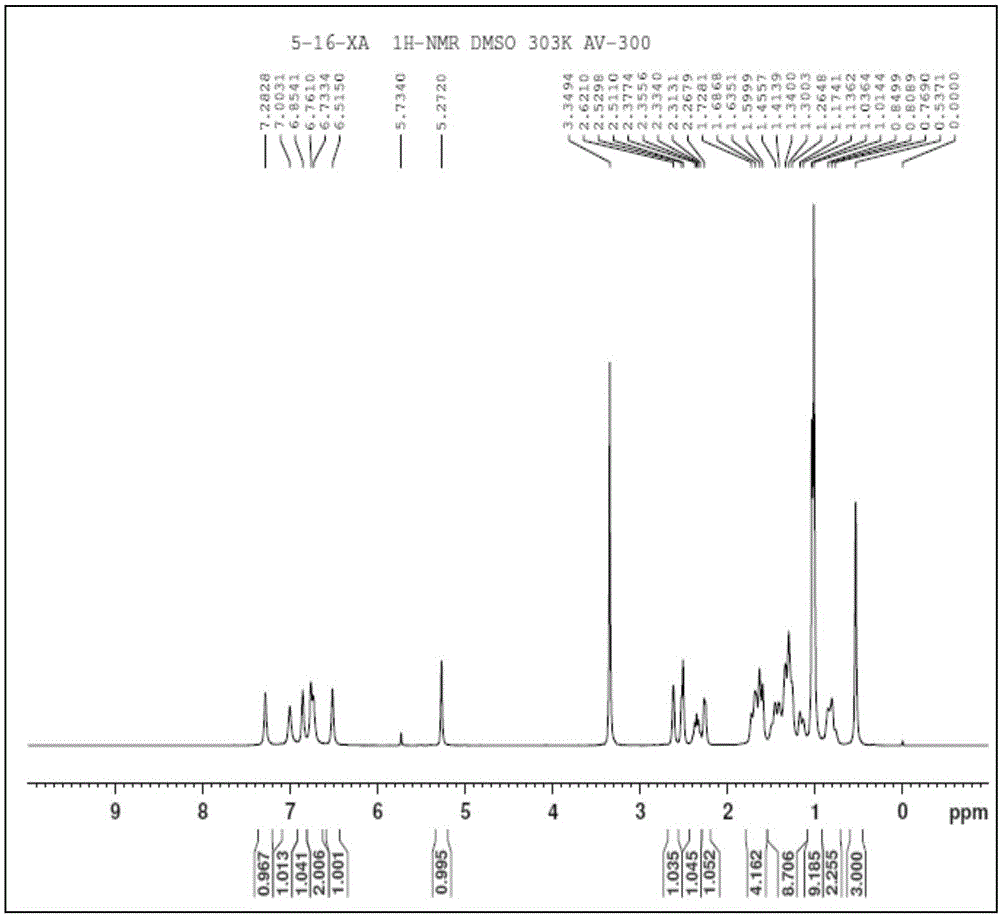
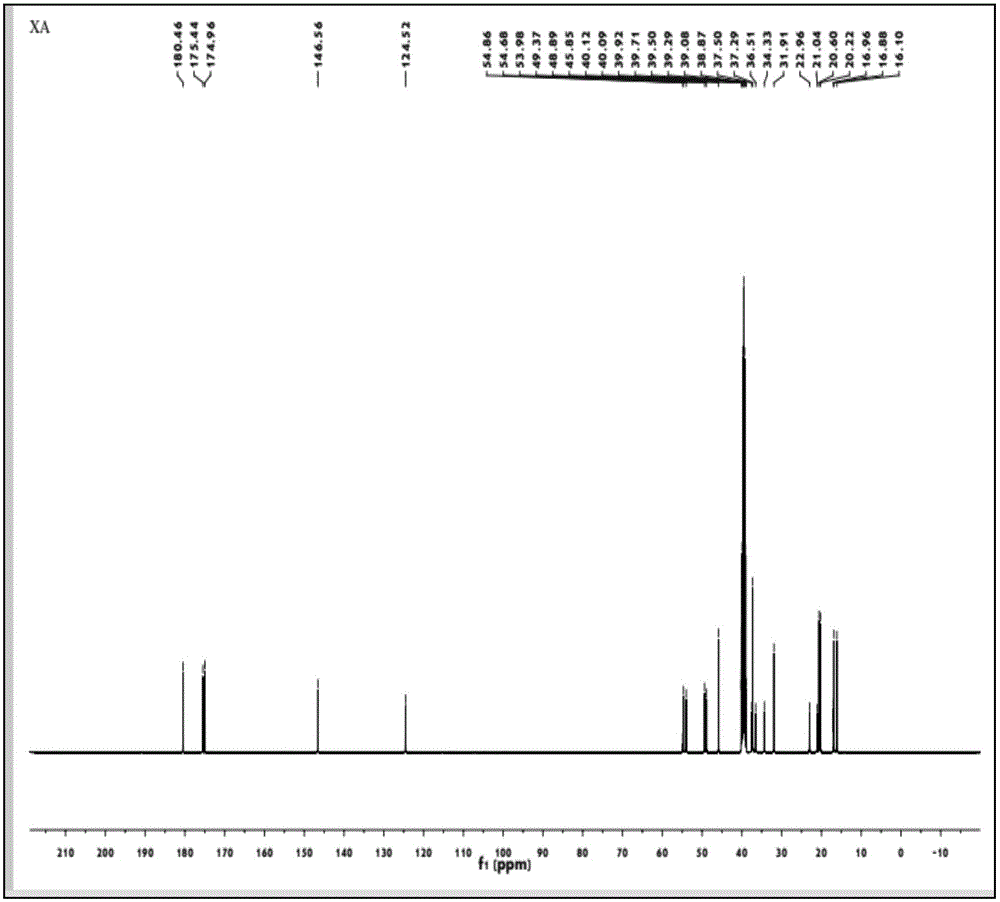
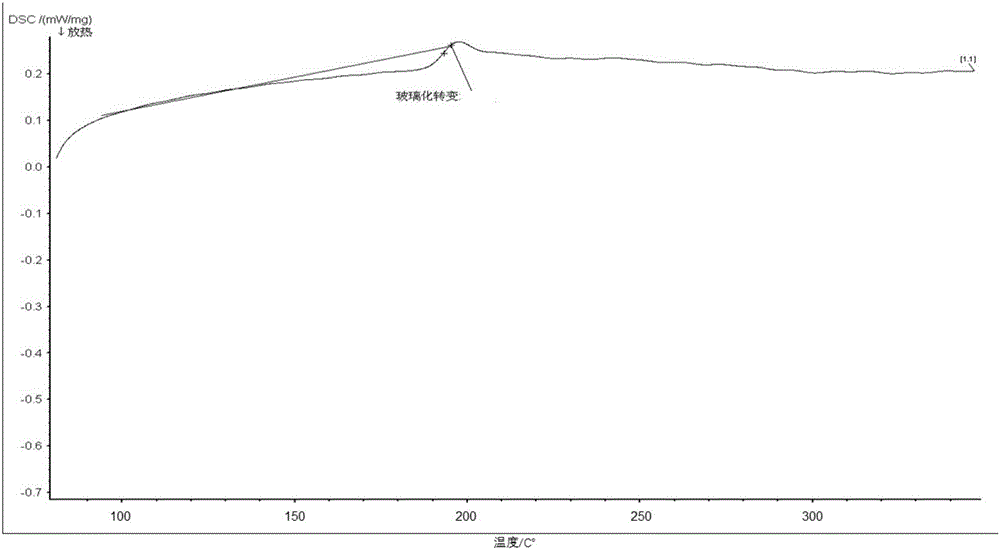
Fumaropimaric acid modified acrylamide compound, preparation method thereof and polymer prepared by fumaropimaric acid modified acrylamide compound
A technology of fumopimaric acid and acrylamide, which is applied in the preparation of carboxylic acid amide, amino compound, organic compound, etc., can solve the problems of poor heat resistance, low recyclability and slow reaction speed of PAM, and achieve Solve the effects of poor heat resistance, convenient operation, and simple reaction device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]Put 10g of fumaric acid in a three-necked flask, dissolve it with 15g of dichloromethane, and add 0.05g of anhydrous pyridine as a catalyst, then slowly add 5.6g of thionyl chloride dropwise to the reaction system at 65°C, and reflux Carry out rotary steaming after reacting 5h to remove excessive anhydrous dichloromethane and sulfur oxychloride, obtain 9.2g fumapimaric acid chloride; 9.2g fumapimaric acid chloride is dissolved in 20g methylene chloride, in salt ice bath condition , and slowly added dropwise to 2.5g of ammonia water, reacted for 6h, and the resulting product was washed and purified to obtain 8.9g of fumapimaric acid amide; 8.9g of fumapimaric acid amide was completely dissolved in ethanol, and added with a mass concentration of 10 % NaOH solution, keep the pH of the system to be 10, then the resulting mixture is added dropwise to 153g mass concentration in the sodium hypobromite solution of 5% under the condition of the ice-salt bath, after the dropwise ad...
Embodiment 2
[0047] Put 10g of fumaric acid in a three-necked flask, dissolve it with 13g of dichloromethane, and add 0.03g of anhydrous pyridine as a catalyst, then slowly add 5.6g of thionyl chloride dropwise to the reaction system at 60°C, and reflux Carry out rotary evaporation after reacting 4h to remove excessive anhydrous dichloromethane and sulfur oxychloride, obtain 8.9g fumapimaric acid chloride; 8.9g fumapimaric acid chloride is dissolved in 18g methylene chloride, in salt ice bath condition , and slowly added dropwise to 2.0g ammonia water, reacted for 5h, washed and purified the resulting product to obtain 8.0g fumapimaric acid amide; 8.0g fumapimaric acid amide was completely dissolved in ethanol, and added with a mass concentration of 10 % NaOH solution to keep the pH of the system at 11, then the resulting mixture is added dropwise to 138g of a 5% sodium hypobromite solution under the condition of an ice-salt bath. After the dropwise addition, the ice-salt bath continued to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com